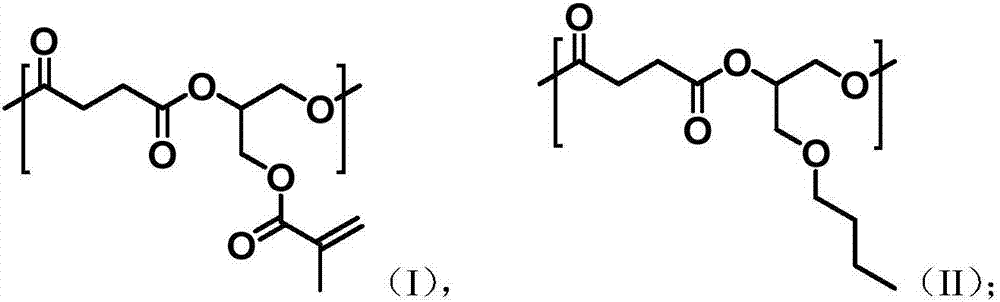
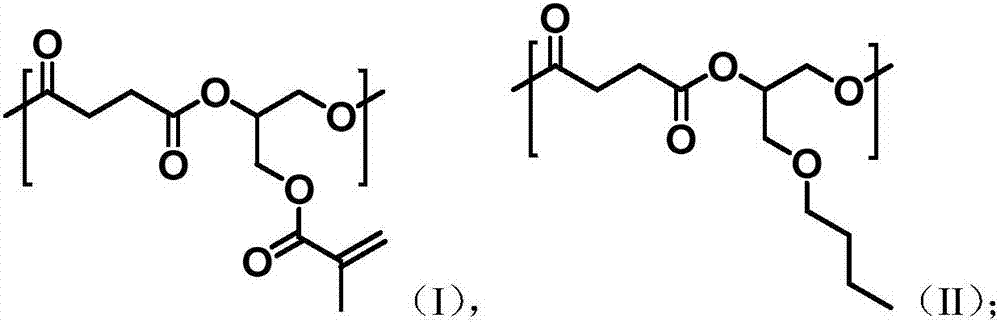
Unsaturated aliphatic polyester as well as preparation method and application thereof
An aliphatic polyester and unsaturated technology, which is applied in the field of medical materials and unsaturated aliphatic polyester, can solve the problems of poor photocuring ability, lack of reactive active sites in the main chain, and low biological activity, and achieve good elastic modulus The effect of volume and swelling properties, good biodegradability, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of unsaturated aliphatic polyester
[0034] Take 10g (100mmol) of succinic anhydride, 7.1g (50mmol) of glycidyl methacrylate, 6.5g (50mmol) of n-butyl glycidyl ether, 0.83g (5mmol) of o-tert-butylhydroquinone and zinc isooctanoate 1.755g (5mmol), add 100mL ethyl acetate: butyl acetate = 1:1 volume ratio mixed solution to dissolve, under the protection of nitrogen, 110 ℃ ring-opening copolymerization reaction for 8h, remove the solvent to obtain unsaturated aliphatic polyester.
[0035] The weight-average molecular weight and number-average molecular weight of the obtained unsaturated aliphatic polyester were measured using Jasco Gulliver system (PU-980, CO-965, RI-930, and UV-1570) gel permeation chromatography. Equipped with polystyrene gel columns (Shodex columns K804, K805, and J806), using THF as eluent, polystyrene as standard calibration, measured at 30°C, the measurement result is: M w =16432, M n = 11255, PDI = 1.46.
[0036] (2) Preparation a...
Embodiment 2
[0065] (1) Preparation of unsaturated aliphatic polyester
[0066] Take succinic anhydride (10g, 100mmol), glycidyl methacrylate (50mmol, 7.1g), n-butyl glycidyl ether (50mmol, 6.5g), o-tert-butylhydroquinone (0.33g, 2mmol) and tetrabutylammonium bromide (0.64g, 2mmol), add 100mL of ethyl acetate: butyl acetate = 1:1 volume ratio mixed solution to dissolve, under the protection of nitrogen, 90 ℃ ring-opening copolymerization reaction for 5h, remove the solvent to get Unsaturated aliphatic polyester.
[0067] Gained unsaturated aliphatic polyester is detected by the method described in embodiment 1, and detection result is: M w =6028, M n =4982, PDI=1.21.
[0068] (2) Preparation and properties of photocurable hydrogel
[0069] (a) Formulation of photocurable hydrogel
[0070] 5 g of unsaturated aliphatic polyester prepared in step 1, 0.5 g of hydroxypropyl methacrylate, 0.5 g of hydroxyethyl methacrylate, 0.8 g of hydroxyethyl acrylate, 1.2 g of N,N-dimethylaminoethyl acr...
Embodiment 3
[0086] (1) Preparation of unsaturated aliphatic polyester
[0087] Take succinic anhydride (10g, 100mmol), glycidyl methacrylate (40mmol, 5.68g), n-butyl glycidyl ether (60mmol, 7.8g), o-tert-butylhydroquinone (1.33g, 8mmol) And zinc acetate (0.98g, 8mmol), add 100mL of ethyl acetate: butyl acetate = 1:1 volume ratio mixed solution to dissolve, under the protection of nitrogen, 120 ℃ ring-opening copolymerization reaction for 12h, remove the solvent to obtain unsaturated aliphatic polyester.
[0088] The obtained unsaturated aliphatic polyester is detected by the method described in Example 1, and the detection result is: M w =21611, M n = 12006, PDI = 1.80.
[0089] (2) Preparation and properties of photocurable hydrogel
[0090] (a) Formulation of photocurable hydrogel
[0091] 5 g of unsaturated aliphatic polyester prepared in step 1, 0.5 g of hydroxypropyl methacrylate, 0.5 g of hydroxyethyl methacrylate, 0.8 g of hydroxyethyl acrylate, 1.2 g of N,N-dimethylaminoethyl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Elastic modulus | aaaaa | aaaaa |
| Elastic modulus | aaaaa | aaaaa |
| Elastic modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com