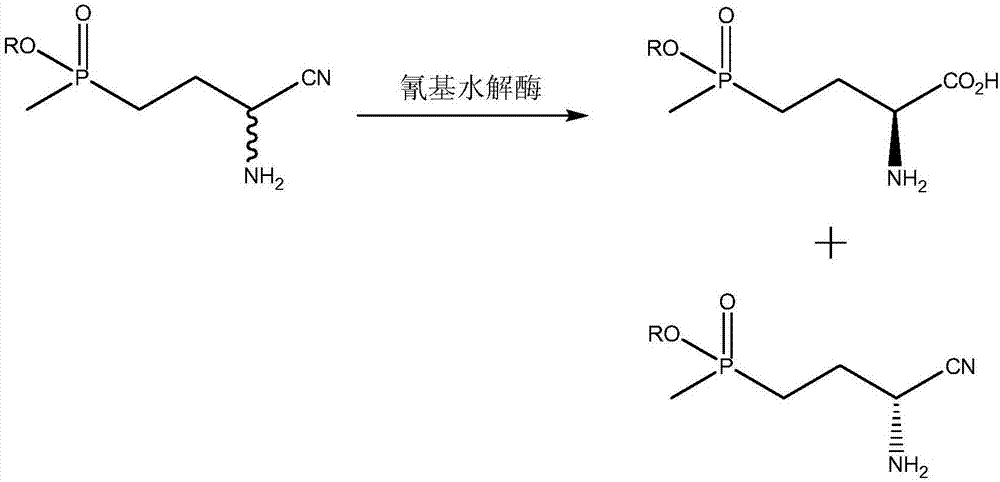
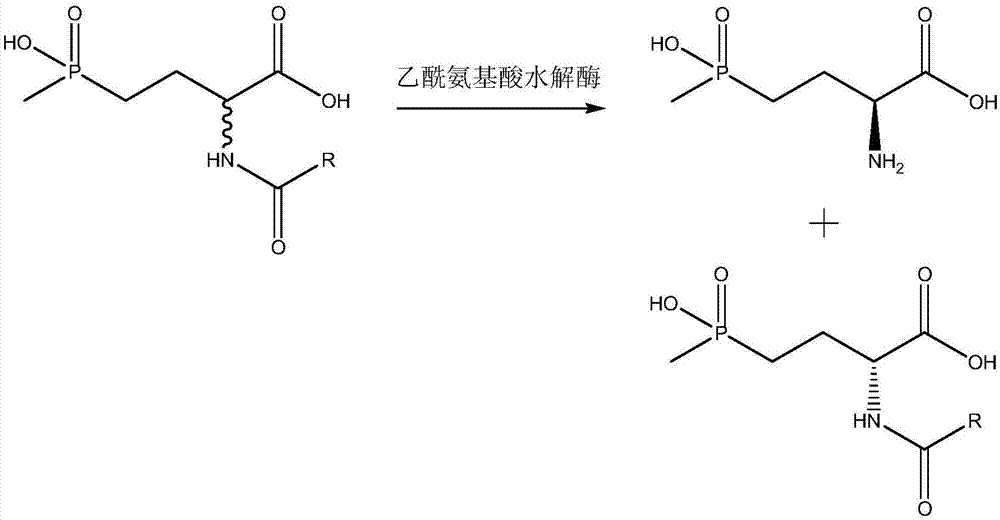
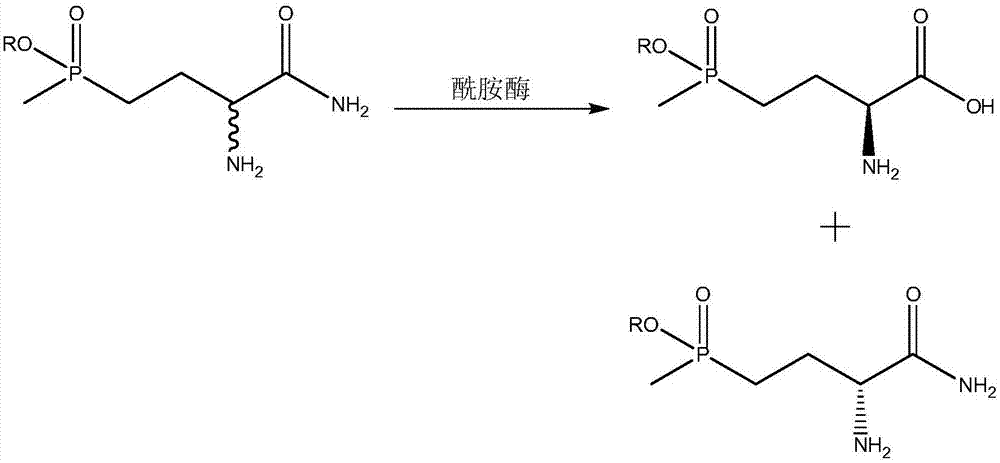
Method for preparing L-phosphinothricin through deracemization and by biological enzyme method
A biological enzyme method and glufosinate-ammonium technology, applied in the biological field, can solve problems such as complex process, soil compaction, and no advantages, and achieve the effect of a low-carbon process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The construction of embodiment 1 genetically engineered bacteria
[0051] 1. Construction and screening of D-amino acid oxidase library and construction of genetically engineered bacteria expressing D-amino acid oxidase
[0052] D-Amino-acid oxidase gene from Rhodotorula gracilis (Rhodotorula gracilis) ATCC26217 by known NCBI accession number AAB51107.1 (Jorge Alonso, et al. D-Amino-acid oxidase gene from Rhodotorula gracilis (Rhodosporidium toruloides) ATCC26217 .Microbiology (1998), 144, 1905-1101), screen the gene sequence with higher similarity with RgDAAO from the NCBI database.
[0053] The gene sequence of the above D-amino acid oxidase, after codon optimization, was sent to Sangon Bioengineering (Shanghai) Co., Ltd. for whole gene synthesis, and cloned into the recombinant expression plasmid pET-28a(+). After the recombinant plasmid was verified to be correct by sequencing, it was transferred into the expression host E. coli BL21 (DE3) for the subsequent expres...
Embodiment 2
[0108] 1. Culture of microorganisms
[0109] Composition of LB liquid medium: peptone 10g / L, yeast powder 5g / L, NaCl 10g / L, dissolved in deionized water and then constant volume, sterilized at 121°C for 20min, ready for use.
[0110] Genetically engineered bacteria E.coli BL21 (DE3) were inoculated into 5 mL LB liquid medium containing 50 μg / mL kanamycin, and cultured with shaking at 37°C for 12 hours. Transfer to 500mL fresh LB liquid medium also containing 50μg / mL Kan, shake culture at 37°C until OD 600 When it reaches about 0.8, add IPTG until its concentration is 0.1-0.3mM, and induce culture at 18-28°C for about 20h. After the cultivation, the culture solution was centrifuged at 10,000 rpm for 10 min, the supernatant was discarded, and the bacterial cells were collected, and stored in a -70°C ultra-low temperature refrigerator until use.
[0111] 2. Preparation of Crude Enzyme Solution
[0112] The bacterial cells collected after the cultivation were washed with 50 mM ...
Embodiment 3
[0121]D-amino acid oxidase derived from Neurospora crassa OR74A, glutamate dehydrogenase derived from Pseudomonas putida KT2440, glucose dehydrogenase derived from Bacillus megaterium DSM319 were selected, and reference example 1 was made for strain construction.
[0122] Collect the cells after microbial culture, obtain the crude enzyme liquid and measure the enzyme activity. For the specific method, refer to Example 2. The crude enzyme liquid activities of the three enzymes are 3.741U / L, 22.17U / L, and 1012.65U / L respectively.
[0123] With 0.1M NH 3 ·NH 4 Cl buffer solution (pH=8.0) was prepared with 100mM D,L-glufosinate-ammonium solution, and 0.1M NH 3 ·NH 4 Prepare 100mM glucose solution in Cl buffer solution (pH=8.0), take 150μL of 100mM D,L-glufosinate-ammonium solution and 180μL of 100mM glucose solution, add them to 2mL EP tube, then add D-amino acid oxidase crude enzyme solution 700 μL, glutamate dehydrogenase crude enzyme solution 165 μL, glucose dehydrogenase cr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pre-denatured | aaaaa | aaaaa |
| Extend | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com