Glutathione response-type dual-drug carrier as well as preparation method and application thereof
A glutathione and dual technology, applied in the field of biomedical engineering materials, can solve the problem that the application of glutathione-responsive hyperbranched polyamidoamine dual drug carrier has not yet been reported, and achieves the promotion of drug synergy and convenient operation. , good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Synthesis of N,N'-bis(acryloyl)cystamine (CBA)
[0060] Dissolve cystamine dihydrochloride and solid sodium hydroxide in pure water respectively, dissolve acryloyl chloride in dichloromethane, and then slowly alternate sodium hydroxide aqueous solution and acryloyl chloride in dichloromethane under ice bath conditions for 20 minutes Add dropwise to the aqueous solution of cystamine dihydrochloride, react at room temperature for 6 hours after the dropwise addition, magnetically stir during the reaction, and the rotation speed is 300rpm; Vacuum-dried at 40°C for 12 hours to obtain N,N'-bis(acryloyl)cystamine (CBA).
[0061] The mol ratio of described cystamine dihydrochloride, sodium hydroxide, acryloyl chloride is 1:2.7:2.5; The volume ratio of dichloromethane and reaction system solution used during the extraction is 20:1; The amount is 4 times by mass of cystamine dihydrochloride, 2.5 times by mass of sodium hydroxide, and the amount of dichloromethane used...
Embodiment 2
[0062] Example 2: Synthesis of N,N'-bis(acryloyl)cystamine (CBA)
[0063] Dissolve cystamine dihydrochloride and solid sodium hydroxide in pure water respectively, dissolve acryloyl chloride in dichloromethane, and then slowly alternate sodium hydroxide aqueous solution and acryloyl chloride in dichloromethane under ice bath conditions for 100 minutes Add dropwise to the aqueous solution of cystamine dihydrochloride, react at room temperature for 12 hours after the dropwise addition, magnetically stir during the reaction, and the rotation speed is 600rpm; Vacuum-dried at 40°C for 24 hours to obtain N,N'-bis(acryloyl)cystamine (CBA).
[0064] The mol ratio of described cystamine dihydrochloride, sodium hydroxide, acryloyl chloride is 1:1.5:1.5; The volume ratio of dichloromethane and reaction system solution used during the extraction is 50:1; The amount is 10 times by mass of cystamine dihydrochloride, 5 times by mass of sodium hydroxide, and the amount of dichloromethane use...
Embodiment 3
[0065] Example 3: NMR characterization of N,N'-bis(acryloyl)cystamine (CBA)
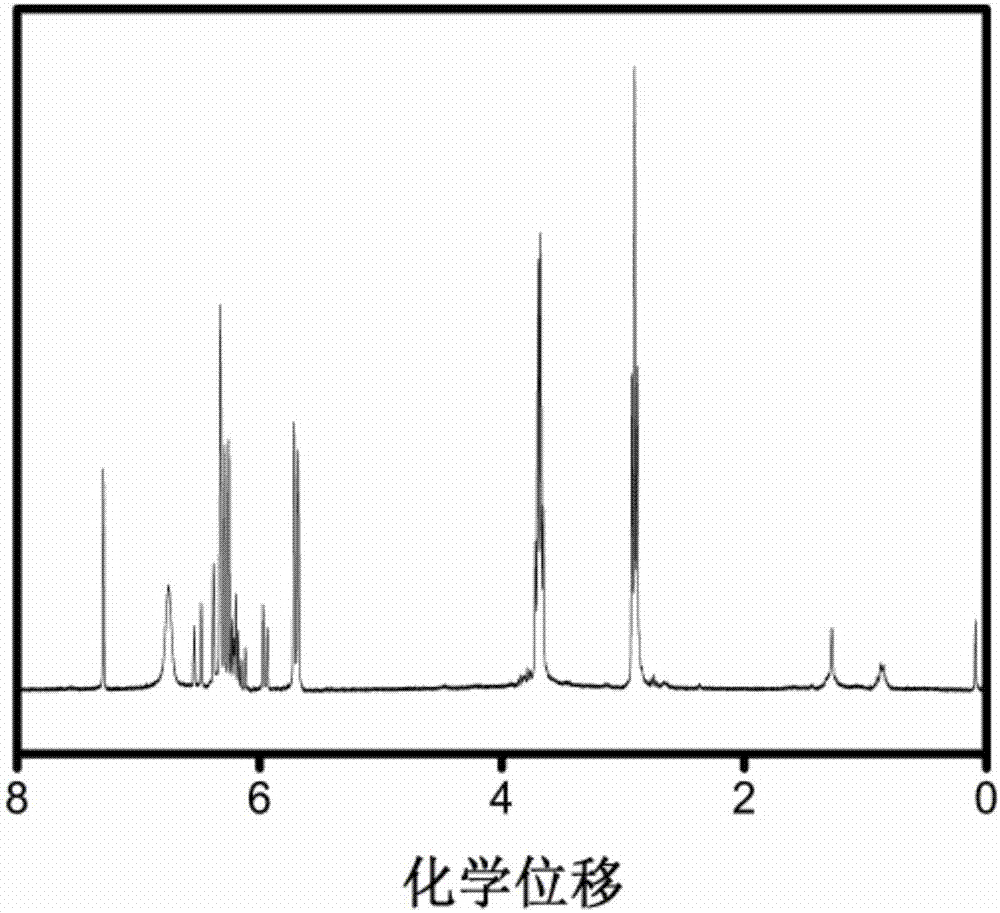
[0066] The N,N'-bis(acryloyl)cystamine (CBA) obtained in Example 1 was dissolved in deuterated chloroform for H NMR characterization. Such as figure 1 As shown, the peaks at 2.92, 3.60, and 6.65 ppm of chemical shifts correspond to the proton peaks of methylene on cystamine dihydrochloride; the peaks at 5.62, 6.25 ppm of chemical shifts correspond to proton peak. figure 1 The results proved that N,N'-bis(acryloyl)cystamine (CBA) was successfully synthesized in this step.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com