Culture method for Schwann cells
A technology for Schwann cells and culture methods, applied in the directions of cell culture active agents, cell culture supports/coatings, animal cells, etc., which can solve problems such as the limitation of collection volume, improve yield and purity, promote adherence and Effects of migration, prevention of agglomeration and multilayer growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: UCB source culture and harvest MSCs
[0067] UCB is derived from UCB mononuclear cells frozen in cord blood banks.
[0068] Resuscitate UCB-MNCs, wash once with normal saline, resuspend cells with 25mL normal saline, carefully pour into 15mL lymphocyte separation medium, 800g, and centrifuge horizontally for 20 minutes; take the middle cloud layer, wash twice with normal saline; culture with MSCs Resuspend the cells and adjust the cell density to 2×10 5 / cm 2 , inoculated to 175cm 2 Culture in tissue culture flasks (product number: EasyFlask, manufacturer: NUNC), change the medium every other day, and harvest when it reaches 70-80% confluence; discard the culture supernatant when harvesting, wash the culture surface once with normal saline, and add 0.25% pancreatic Protease solution, digest at room temperature for 5 minutes, add 1mL aprotinin solution to stop the digestion; centrifuge at 400g for 5min, discard the supernatant, and harvest the precipitate; ...
Embodiment 2
[0070] Example 2: UCB-MSCs source culture and harvest neurospheres
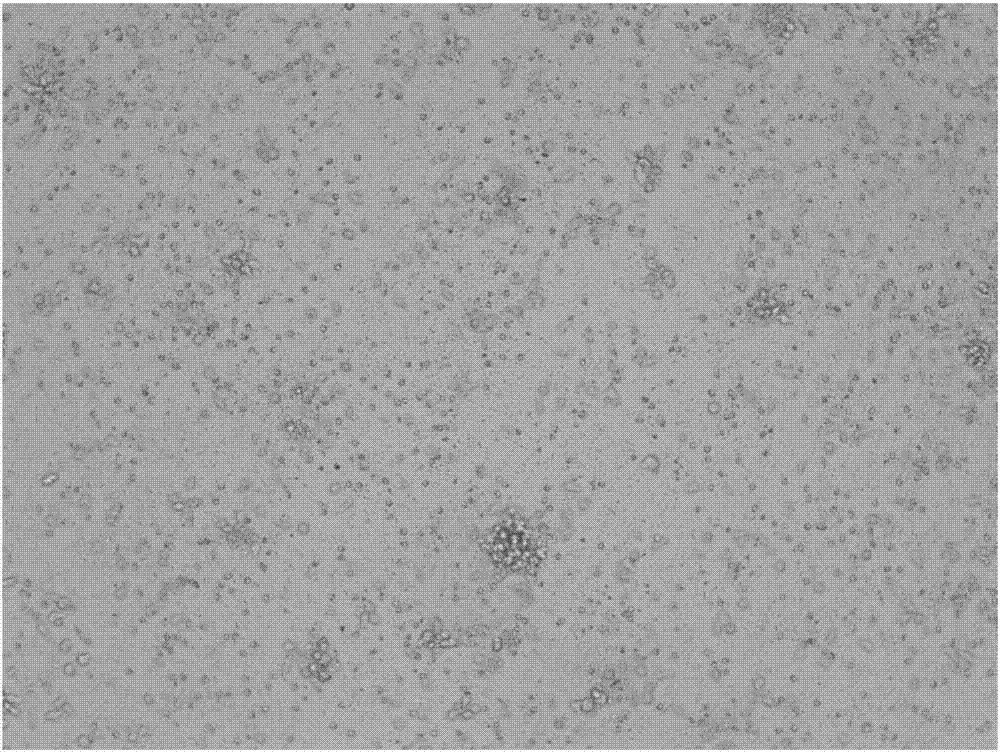
[0071] Resuspend P1 passage UCB-MSCs in neurosphere medium, adjust the cell density to 2×10 5 / mL, inoculated into bacterial culture dishes (product number: 4021, manufacturer: NUNC), 20mL per dish, 37°C, 5% CO 2 , cultured under saturated humidity conditions; add 10mL of new neurosphere medium every other day, continue to culture for 7 days, and subculture; when subculture, gently pipette the culture medium, centrifuge at 300g for 10 minutes, resuspend the pellet with neurosphere medium, and gently Pipette 10 times to make the neurospheres form 3-5 cell clusters or single cells, subculture in a 1:5 split plate; harvest P3 generation neurospheres.
[0072] Wherein, the neurosphere culture medium is neurobasal medium comprising the following substances:
[0073] 0.5 mM L-Glutamine;
[0074] 2% B-27™ Additive;
[0075] 20ng / mL recombinant human basic fibroblast growth factor;
[0076] 20ng / mL recombinant h...
Embodiment 3
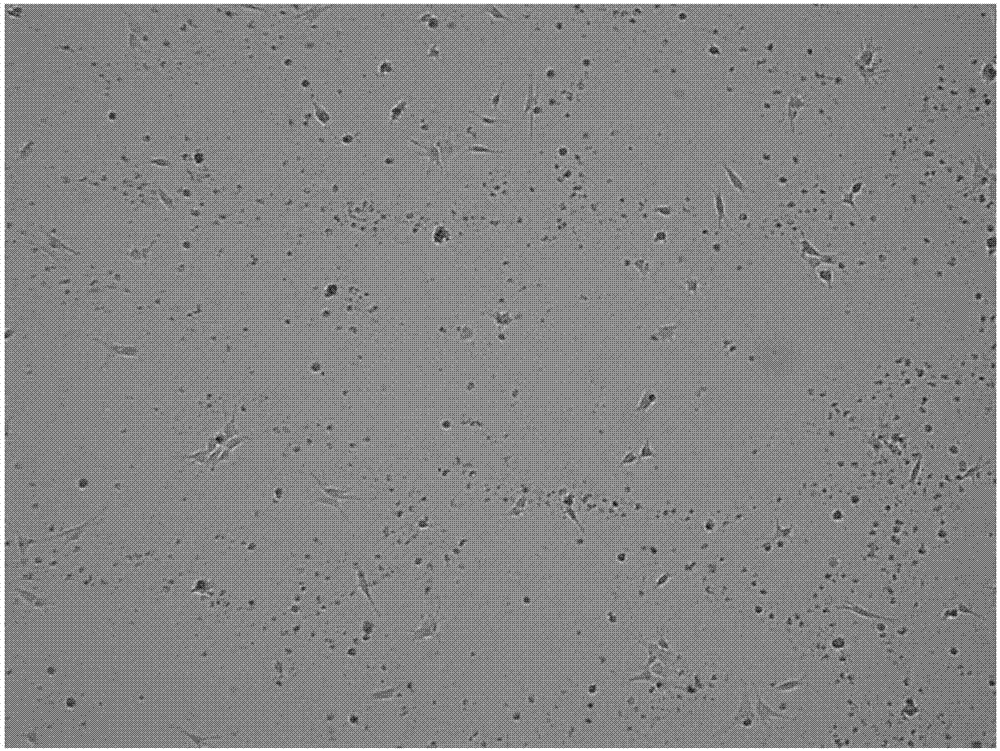
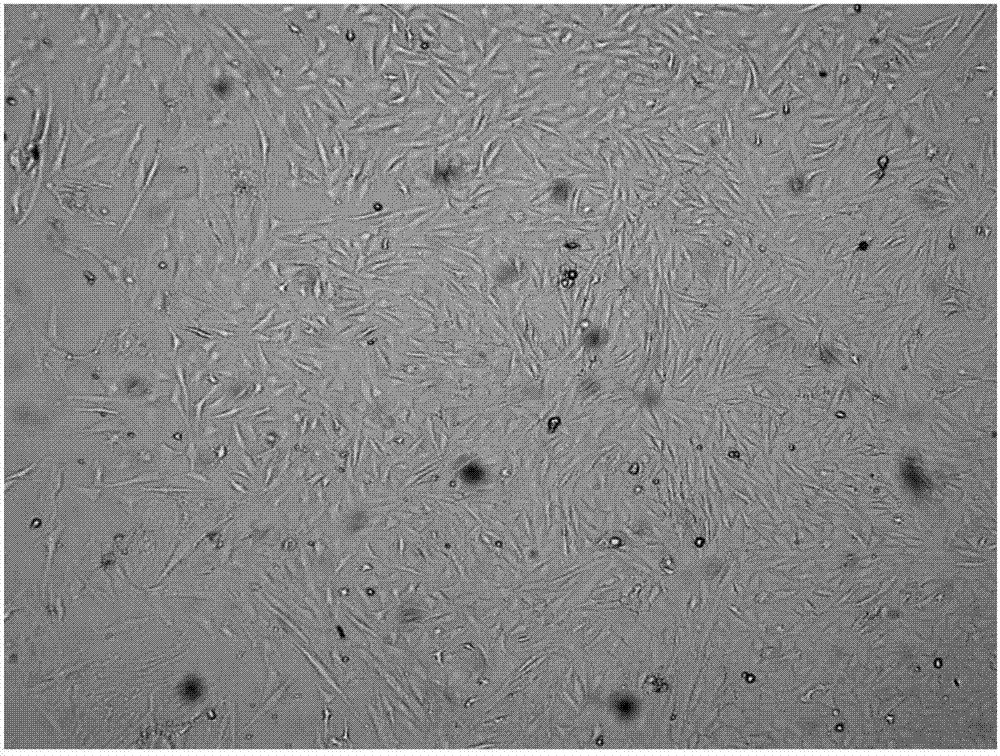
[0077] Example 3: culturing and harvesting Schwann cells
[0078] Suspend P3 generation neurospheres with Schwann cell culture medium, inoculate them into tissue culture flasks coated with recombinant human laminin, recombinant human fibronectin, and recombinant human heregulin-β1, change the medium every other day, 8-12 When 60-80% confluence, discard the culture supernatant, wash the culture surface once with PBS, add 1mL of AccutaseTM digestion solution, digest at room temperature for 5 minutes, add 200uL aprotinin solution to stop the digestion; centrifuge at 400g for 5min, discard the supernatant, The precipitated cells were harvested and recorded as Schwann cells derived from UCB at generation P0. P0 generation Schwann cells were subcultured once.
[0079] Wherein, the Schwann cell culture medium is neurobasal medium comprising the following substances:
[0080] 0.5 mM L-Glutamine,
[0081] 2% B-27™ Additive,
[0082] 10% Animal Component Free Serum Replacement,
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com