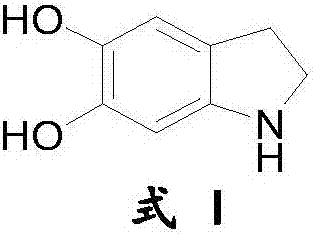
Preparation method of compound 5,6-dihydroxy indoline and halogen acid salts thereof
A technology of oxindoline and hydrohalide, which is applied in the field of compound 5, can solve problems such as being unsuitable for industrial production, and achieve the effects of good stability, less side reactions, and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
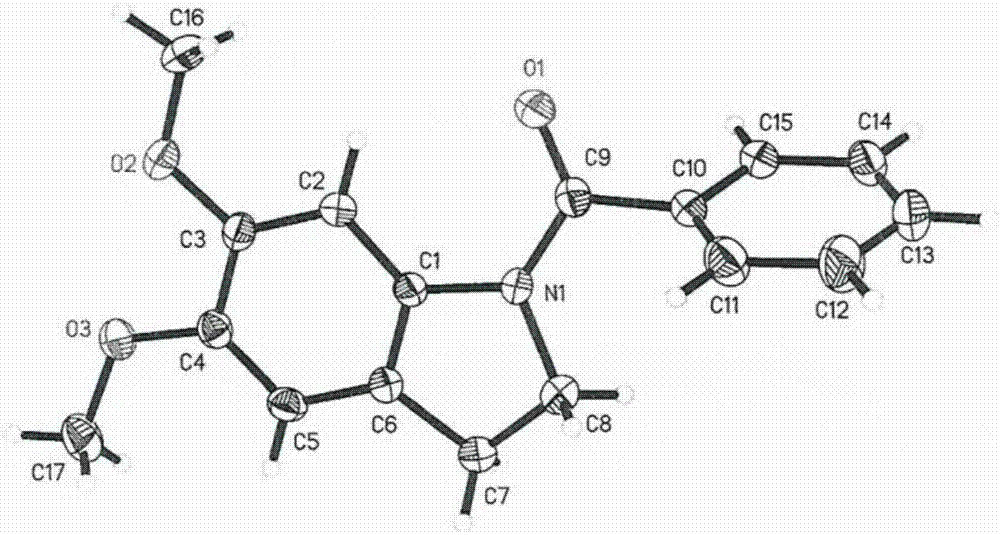
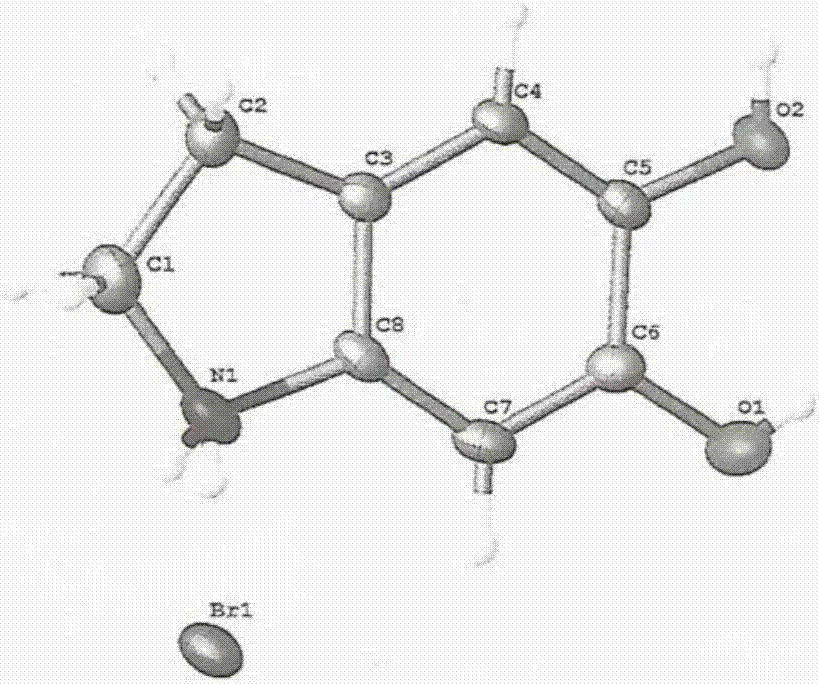
Image
Examples
Embodiment 1
[0049] A kind of preparation method of 5,6-dihydroxyindoline, comprises the steps:
[0050] 1) Synthesize amide SM-1 with 3,4-dimethoxyethylamine and acetyl chloride as raw materials, the chemical equation is:
[0051]
[0052] The specific operation process is as follows: install mechanical stirring, constant pressure dropping funnel and built-in thermometer on a 1L three-neck round bottom flask; the outside is ethanol circulating cooling liquid. 90.56g (0.5mol) of 3,4-dimethoxyphenethylamine, 100mL (0.72mol) of triethylamine and 455mL of dichloromethane were sequentially added into the reaction flask. Under the condition of stirring, the above mixed solution was lowered to 0°C. Then, measure 0.60 mol of acetyl chloride or acetic anhydride, and transfer it to a constant pressure dropping funnel; at an internal temperature of 0°C, drop acetyl chloride or acetic anhydride into the above reaction solution; the dropping time lasts about 20-30 minutes . After the dropwise ad...
Embodiment 2
[0070] A kind of preparation method of 5,6-dihydroxyindoline hydrobromide, comprises the steps:
[0071] 1) Synthesize amide SM-1 with 3,4-dimethoxyethylamine and acetyl chloride as raw materials, the chemical equation is:
[0072]
[0073] The specific operation process is as follows: install mechanical stirring, constant pressure dropping funnel and built-in thermometer on a 1L three-neck round bottom flask; the outside is ethanol circulating cooling liquid. 90.56g (0.5mol) of 3,4-dimethoxyphenethylamine, 100mL (0.72mol) of triethylamine and 455mL of dichloromethane were sequentially added into the reaction flask. Under the condition of stirring, the above mixed solution was lowered to -5°C. Then, measure 0.60 mol of acetyl chloride or acetic anhydride, and transfer it to a constant pressure dropping funnel; at an internal temperature of 0°C, drop acetyl chloride or acetic anhydride into the above reaction solution; the dropping time lasts about 20-30 minutes . After t...
Embodiment 3
[0088] A kind of preparation method of 5,6-dihydroxyindoline, comprises the steps:
[0089] 1) Using 3,4-dimethoxyethylamine and tert-valeryl chloride as raw materials to synthesize amide SM-1, the chemical equation is:
[0090]
[0091] The specific operation process is as follows: install mechanical stirring, constant pressure dropping funnel and built-in thermometer on a 1L three-neck round bottom flask; the outside is ethanol circulating cooling liquid. 90.56g (0.5mol) of 3,4-dimethoxyphenethylamine, 100mL (0.72mol) of triethylamine and 455mL of dichloromethane were sequentially added into the reaction flask. Under the condition of stirring, the above mixed solution was lowered to 0°C. Then, measure 0.6 mol of t-valeryl chloride and transfer it to a constant pressure dropping funnel; add t-valeryl chloride dropwise to the above reaction solution at an internal temperature of 0°C; the dropping time lasts about 20-30 minutes. After the dropwise addition, react at 0° C. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com