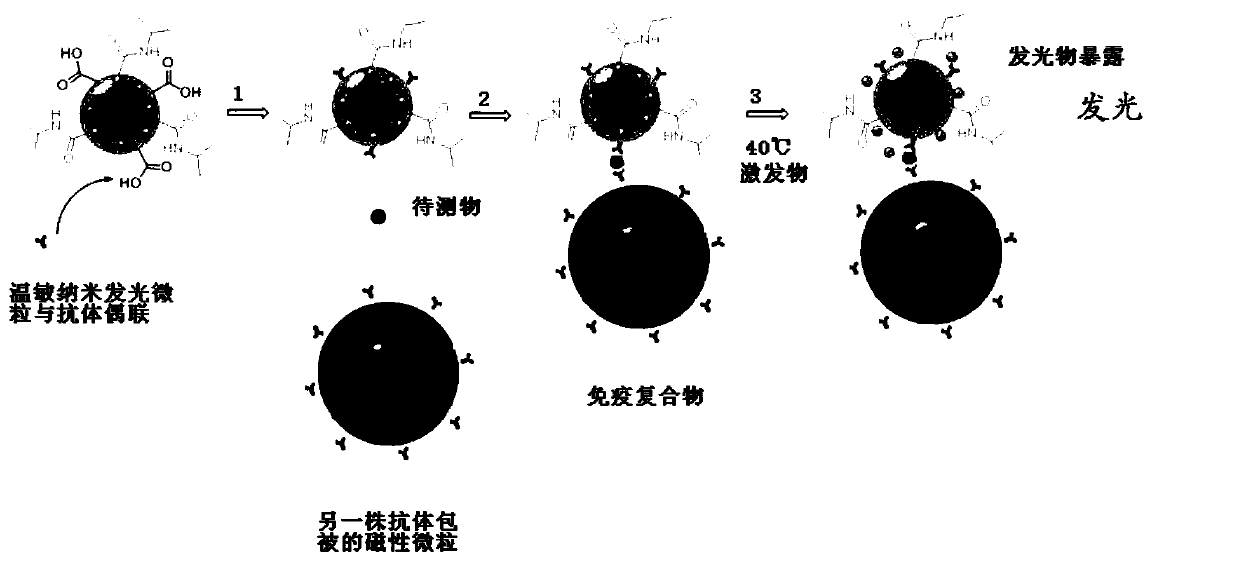
A chemiluminescent immunoassay based on intelligent nanoluminescent particle labeling amplification and its application
A technology of chemiluminescence immunity and luminescence particles, which is applied in the direction of chemical reaction of materials for analysis, chemiluminescence/bioluminescence, analysis of materials, etc., which can solve the problem of limited number of coupling functional groups, limited labeling amplification effect, and low labeling efficiency. and other problems, to achieve the effects of high sensitivity, improved labeling efficiency of luminescent molecules and antibodies, and a wide detection range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1. Preparation of temperature-sensitive nano luminescent particles:
[0036] Preparation of A.P (NIPAm-co-MAA) nanoparticles
[0037] Preparation of isopropylacrylamide and methacrylic acid copolymer nanoparticles (P(NIPAm-co-MAA)) by emulsion polymerization: 50mg of sodium dodecyl sulfate (SDS) and 100mg of permeate were added to a three-necked flask. Potassium sulfate, add 100mL deionized water to dissolve. Take 2 mL of monomer isopropyl acrylamide, 0.1 mL of methacrylic acid and 0.05 mL of cross-linking agent methylene bis acrylamide (MBA), mix it well in advance, add it to a three-necked flask, emulsify it and stir at 70°C The reaction was heated for 8 hours. After the product was collected, the liquid was removed by centrifugation (rotating speed 10000 rpm, centrifugation for 30 min), and then resuspended in deionized water. This was repeated 3 times to obtain P(NIPAm-co-MAA) nanoparticles.
[0038] Preparation of B.P (NIPAm-co-MAA) Nanoparticle / Acridinium Ester Lumine...
Embodiment 2
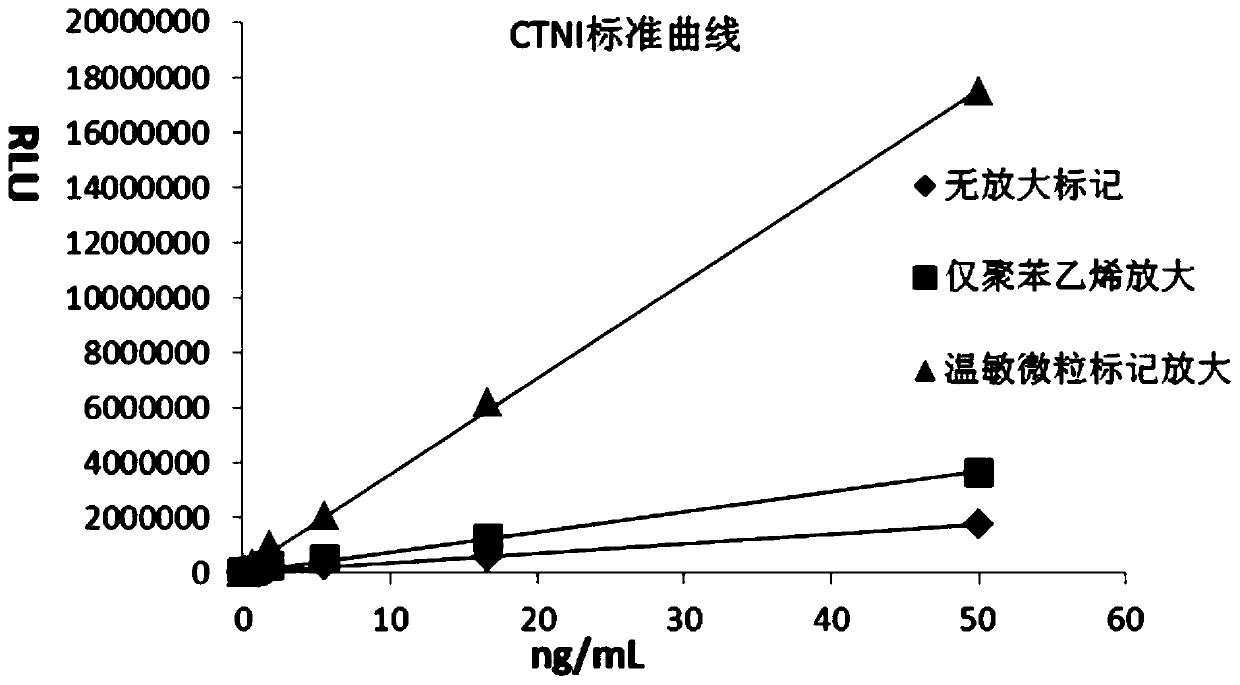
[0055] In order to verify the significant advantages of the temperature-sensitive nano-luminescent particle labeling amplification system of the present invention, we designed the following parallel comparative experiments. The concentration gradient of the CTNI standard substance of the same concentration is detected separately by the traditional double antibody sandwich system, the labeling amplification system using only polystyrene nanospheres, and the labeling amplification system of the present invention, and the luminescence value is recorded to fit the standard curve. The concentrations of CTNI detection antibodies used in the above three systems are equal.
[0056] A. Traditional CLIA double antibody sandwich method:
[0057] It was diluted with PBS buffer to prepare CTNI standards with concentration gradients of 0, 0.003, 0.008, 0.023, 0.069, 0.206, 0.617, 1.852, 5.556, 16.667, and 50.000 ng / mL. In the reaction cup, add 10 μL of streptavidin-coated magnetic microspheres,...
Embodiment 3
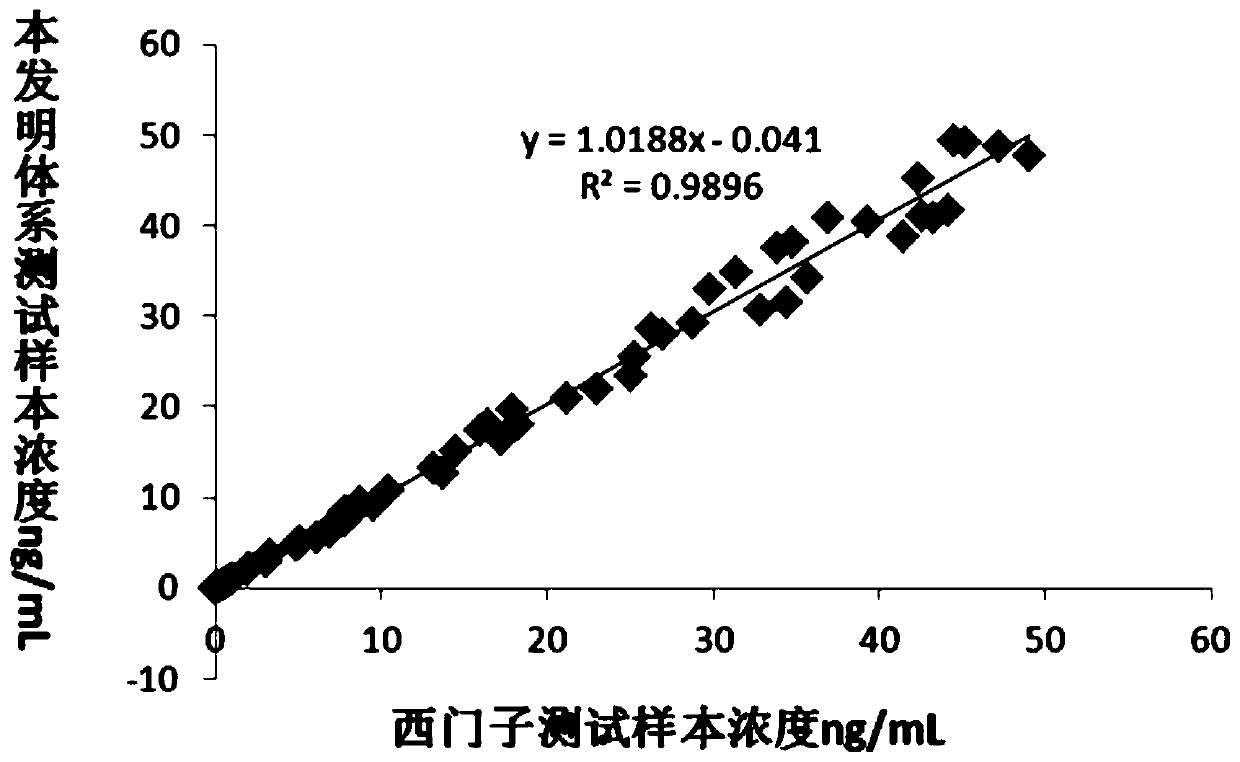
[0070] Example 3 The system of the present invention tests the correlation between the CTNI concentration in the serum sample and the Siemens measurement value.
[0071] In order to verify the accuracy of the system kit of the present invention, a plurality of serum samples were tested for CTNI content with Siemens CTNI chemiluminescence detection reagent, and 60 of them were selected with a suitable gradient. The system of the present invention was used to determine the content of CTNI, and the correlation was compared by plotting. by image 3 It can be seen that the two systems show good correlation, R 2 The value is as high as 0.9896. It is confirmed that the system of the present invention and the kit based on the system have excellent accuracy in addition to ultra-high sensitivity and wider detection range.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com