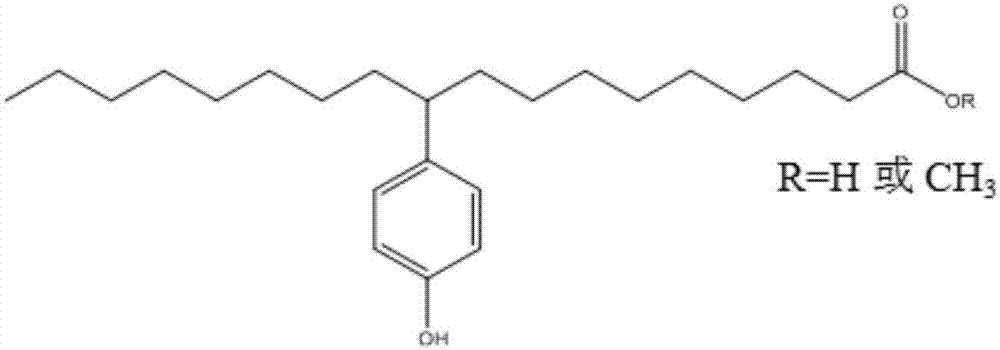
Phenolic hydroxyl fatty acid antioxidant and preparation method and application thereof
A technology of phenolic hydroxy fatty acid and antioxidant, applied in the field of phenolic hydroxy fatty acid antioxidant and its preparation, can solve the problems of low antioxidant efficiency, poor antibacterial effect, poor thermal stability, etc., and achieve high antioxidative efficiency and antibacterial effect Good, good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
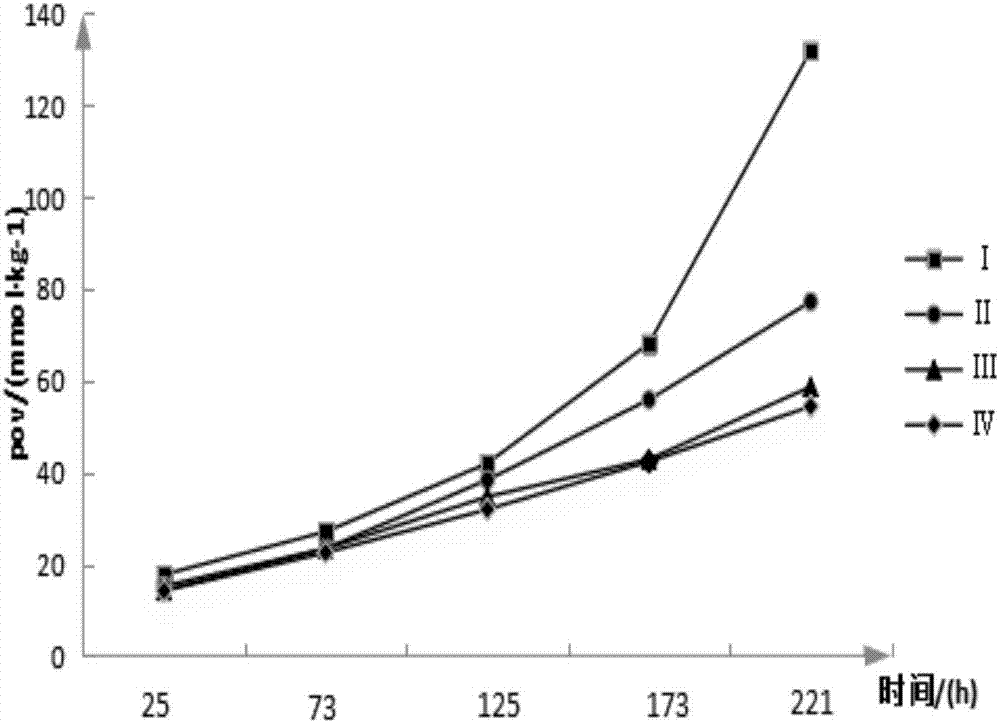
Image
Examples
Embodiment 1
[0030] A preparation method and application thereof of phenolic hydroxyl fatty acid antioxidant, comprising the following steps:
[0031] (1) Add 1.2g montmorillonite, 40g methyl oleate, and 19.236g phenol (the molar ratio of methyl oleate to phenol is 1:2) into the autoclave, seal it, and fill it with argon inert gas to 0.4Mpa, Heated to 200°C and reacted at this temperature for 6h to obtain a mixture containing isomerized products.
[0032] (2) After the mixture was cooled, it was washed with ethyl acetate, and the solid catalyst was removed by suction filtration. The obtained filtrate was added with deionized water and rotary evaporated at 90°C.
[0033] (3) Take 5g of the remaining liquid obtained after rotary steaming and put it into a high-pressure reactor, add 1.5g of Pd / C hydrogenation catalyst, dilute it with 25ml of methanol, seal it, fill it with hydrogen to 0.4 Mpa, react at room temperature for 4 hours, and then use acetic acid Wash with ethyl ester, remove the h...
Embodiment 2
[0037] A preparation method and application thereof of phenolic hydroxyl fatty acid antioxidant, comprising the following steps:
[0038] (1) Add 2.8g montmorillonite, 40g methyl oleate, and 19.236g phenol (the molar ratio of methyl oleate to phenol is 1:2) into the autoclave, seal it, fill it with nitrogen inert gas to 0.4Mpa, and heat To 200 ° C, and react at this temperature for 6h to obtain a mixture containing isomerization products.
[0039] (2) After the mixture was cooled, it was washed with ethyl acetate, and the solid catalyst was removed by suction filtration. The obtained filtrate was added with deionized water and rotary evaporated at 90°C.
[0040] (3) Take 5g of the remaining liquid obtained after rotary steaming and put it into a high-pressure reactor, add 1.5g of Pd / C hydrogenation catalyst, dilute with 25ml of methanol, seal it, fill it with hydrogen to 0.4Mpa, react at room temperature for 4 hours, and then use ethyl acetate The ester was washed, the hydrog...
Embodiment 3
[0044] A preparation method and application thereof of phenolic hydroxyl fatty acid antioxidant, comprising the following steps:
[0045] (1) Add 2.8g montmorillonite, 40g methyl oleate, and 19.236g phenol ((the molar ratio of methyl oleate to phenol is 1:2)) into the autoclave, seal it, and fill it with nitrogen inert gas to 0.4Mpa , heated to 200°C, and reacted at this temperature for 8h to obtain a mixture containing isomerized products.
[0046](2) After the mixture was cooled, it was washed with ethyl acetate, and the solid catalyst was removed by suction filtration. The obtained filtrate was added with deionized water and rotary evaporated at 90°C.
[0047] (3) Take 5g of the remaining liquid obtained after rotary steaming and put it into a high-pressure reactor, add 1.5g of Pd / C hydrogenation catalyst, dilute with 25ml of methanol, seal it, fill it with hydrogen to 0.4Mpa, react at room temperature for 4 hours, and then use ethyl acetate The ester was washed, the hydro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| peroxide value | aaaaa | aaaaa |
| peroxide value | aaaaa | aaaaa |
| peroxide value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com