Methods of preparing organic boron compound and beta-carbonyl compound by catalyzing copper ion loaded chitosan microspheres
A technology of chitosan microspheres and hydroxyl compounds, which is applied in the preparation of organic compounds, the separation/purification of carbonyl compounds, and the preparation of carbon-based compounds, etc. It can solve the problems of no continuous production, complex process routes, and complicated operation processes. , to achieve the effect of good stability, wide applicability and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
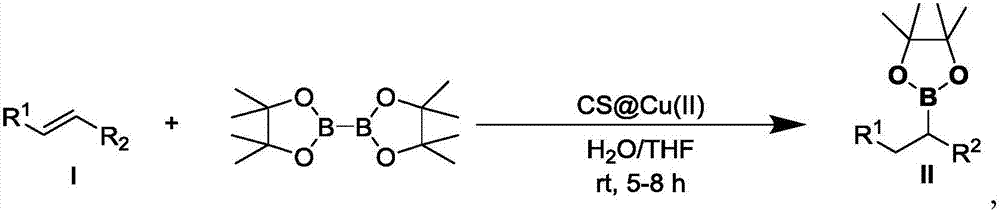
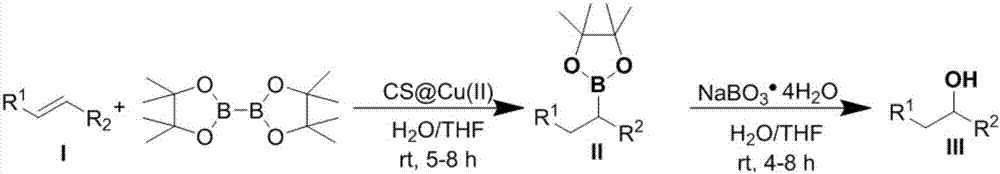
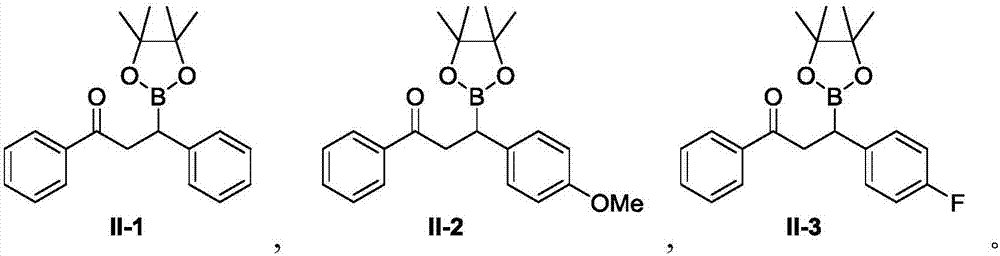
[0048] A preparation method of organoboron compound II-1, the steps are:
[0049] A. Add copper ion-loaded chitosan microspheres (CS@CuSO 4 ) 0.002mmol (calculated based on the amount of copper ion substances loaded, the loading capacity is 15-25wt%, the same below), and add 1.0mL tetrahydrofuran and 1.0mL water, and stir at room temperature (20-25°C, the same below) for 1 hour ;
[0050] B. To the above system, successively add α,β-unsaturated carbonyl compound I-1 (41.6mg, 0.2mmol) and biboronic acid pinacol ester (B 2 (pin) 2 ) (60.9 mg, 2.4 mmol);
[0051] C. The whole reaction system was stirred and reacted at room temperature, and the reaction time was 5 hours;
[0052] D. After the reaction is over, filter the entire reaction system, wash with ethyl acetate 10mL, then extract with ethyl acetate (3×10mL), separate the organic phase, and wash with anhydrous Na 2 SO 4 Dry, filter and remove solvent by rotary evaporation. The residue was purified by column chromatogr...
Embodiment 2
[0058] A preparation method of organoboron compound II-2, the steps are:
[0059] A. Add copper ion-loaded chitosan microspheres (CS@CuSO 4 ) 0.002mmol, and added 1.0mL tetrahydrofuran and 1.0mL water, stirred at room temperature (20-25°C, the same below) for 1 hour;
[0060] B. To the above system, add α,β-unsaturated carbonyl compound I-2 (47.7mg, 0.2mmol) and biboronic acid pinacol ester (B 2 (pin) 2 ) (60.9 mg, 2.4 mmol);
[0061] C. The whole reaction system was stirred and reacted at room temperature, and the reaction time was 5 hours;
[0062] D. After the reaction is over, filter the entire reaction system, wash with ethyl acetate 10mL, then extract with ethyl acetate (3×10mL), separate the organic phase, and wash with anhydrous Na 2 SO 4 Dry, filter and remove solvent by rotary evaporation. The residue was purified by column chromatography with ethyl acetate / petroleum ether mixed solvent=9:1 to obtain II-2 light yellow solid, 72.5 mg, yield 99%.
[0063] The H ...
Embodiment 3
[0068] A preparation method of organoboron compound II-3, the steps are:
[0069] A. Add copper ion-loaded chitosan microspheres (CS@CuSO 4 ) 0.002mmol, and added 1.0mL tetrahydrofuran and 1.0mL water, stirred at room temperature (20-25°C, the same below) for 1 hour;
[0070] B. To the above system, add α, β-unsaturated carbonyl compound I-3 (45.3 mg, 0.2 mmol) and biboronic acid pinacol ester (B 2 (pin) 2 ) (60.9 mg, 2.4 mmol);
[0071] C. The whole reaction system was stirred and reacted at room temperature, and the reaction time was 5 hours;
[0072] D. After the reaction is over, filter the entire reaction system, wash with ethyl acetate 10mL, then extract with ethyl acetate (3×10mL), separate the organic phase, and wash with anhydrous Na 2 SO 4 Dry, filter and remove solvent by rotary evaporation. The residue was purified by column chromatography with ethyl acetate / petroleum ether mixed solvent=9:1 to obtain 69.4 mg of II-3 with a yield of 98%.
[0073] The H NMR a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com