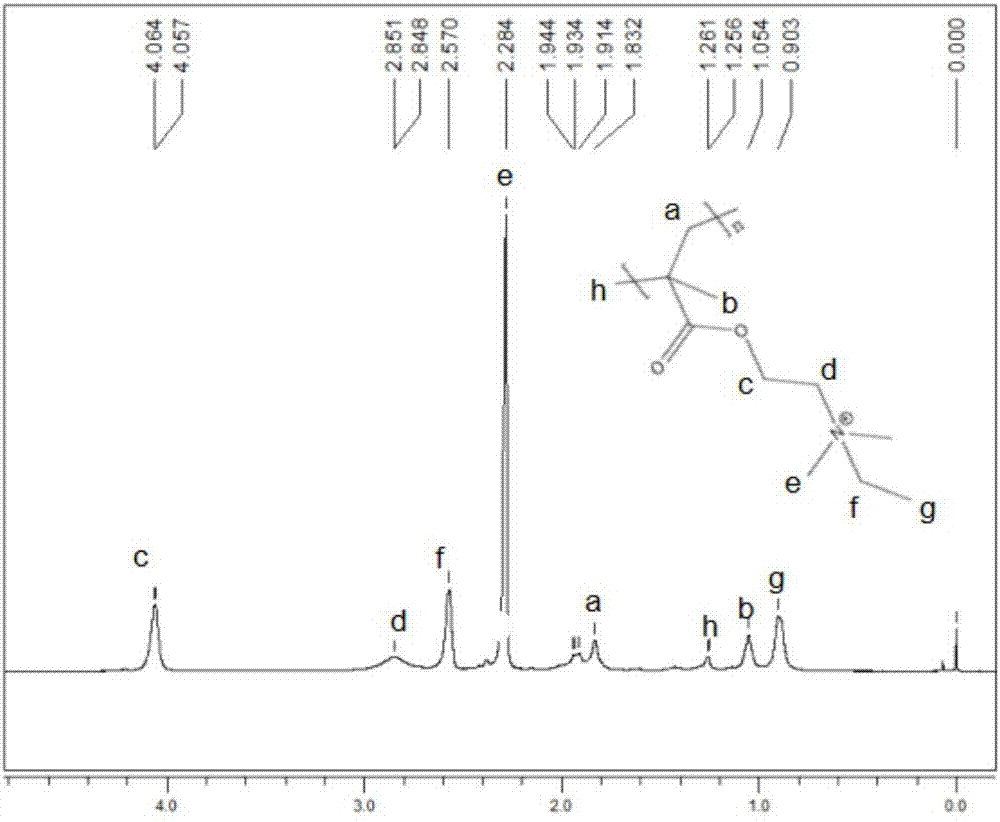
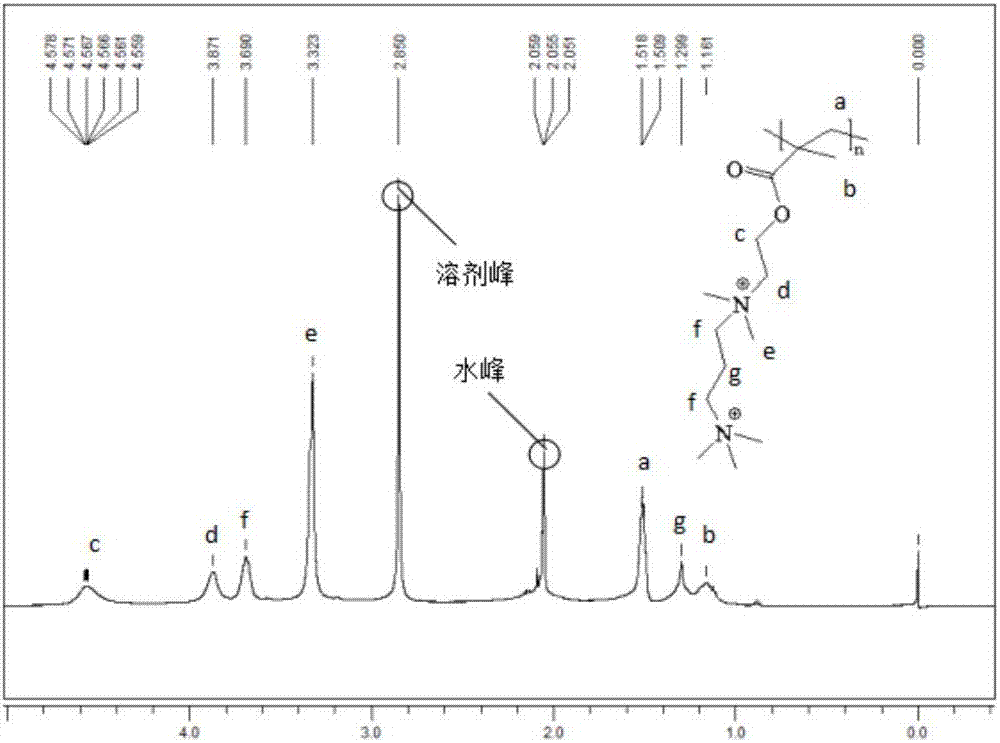
Three-arm branched polymerized ionic liquid gel electrolyte and preparation method thereof
A technology for polymerizing ionic liquids and gel electrolytes, applied in circuits, fuel cells, electrical components, etc., can solve the problems of poor stability, low electrical conductivity, insufficient mechanical strength, etc., and achieve the effects of easy processing and size control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: (R 1 for R 2 for )
[0045] Under nitrogen, 2.68g of trimethylolpropane was dissolved in tetrahydrofuran, cooled in an ice bath, 27.6g of 2-bromoisobutyryl bromide and 12g of triethylamine were successively added dropwise, stirred for 24h and raised to room temperature. The product was transferred to a separatory funnel and extracted with dichloromethane using 10% hydrochloric acid, 5% NaHCO 3 Wash with aqueous solution and deionized water repeatedly. The organic phase was dried over anhydrous magnesium sulfate, and spin-dried under reduced pressure. A mixed solvent of dichloromethane and methanol is used for recrystallization to obtain a three-arm macromolecular initiator.
[0046] Under nitrogen, dissolve 0.037g of cuprous bromide, 4g of dimethylaminoethyl methacrylate and 0.044g of N,N,N',N",N"-pentamethyldiethylenetriamine in 6g of ionized water. Dissolve 0.025 g of the three-arm macromolecular initiator obtained in step 1 in 1 mL of DMSO, and ...
Embodiment 2
[0053] Embodiment 2: (R 1 for R 3 for )
[0054] Under the protection of nitrogen, 2.68g of trimethylolpropane was dissolved in tetrahydrofuran, cooled in an ice bath, 27.6g of 2-bromoisobutyryl bromide and 12g of anhydrous triethylamine were added dropwise, stirred for 24h and raised to room temperature. The product was transferred to a separatory funnel and extracted with dichloromethane using 10% hydrochloric acid, 5% NaHCO 3 Wash with aqueous solution and deionized water repeatedly. The organic phase was dried over anhydrous magnesium sulfate, and spin-dried under reduced pressure. A mixed solvent of dichloromethane and methanol is used for recrystallization to obtain a three-arm macromolecular initiator.
[0055] Under nitrogen, 0.037g of cuprous bromide, 4g of dimethylaminoethyl methacrylate and 0.058g of 1,1,4,7,10,10-hexamethyltriethylenetetramine were dissolved in 6g of deionized water. Dissolve 0.025 g of the three-arm macroinitiator obtained in step 1 in 1 ...
Embodiment 3
[0062] Embodiment 3: (R 1 for R 2 for )
[0063] Under the protection of nitrogen, 2.68g of trimethylolpropane was dissolved in tetrahydrofuran, cooled in an ice bath, 27.6g of 2-bromoisobutyryl bromide and 12g of anhydrous triethylamine were added dropwise, stirred for 24h and raised to room temperature. The product was transferred to a separatory funnel and extracted with dichloromethane using 10% hydrochloric acid, 5% NaHCO 3 Wash with aqueous solution and deionized water repeatedly. The organic phase was dried over anhydrous magnesium sulfate, and spin-dried under reduced pressure. A mixed solvent of dichloromethane and methanol is used for recrystallization to obtain a three-arm macromolecular initiator.
[0064] Under nitrogen, 0.037g of cuprous bromide, 4g of dimethylaminoethyl methacrylate and 0.04g of 2,2-bipyridyl were dissolved in 6g of toluene. Dissolve 0.025 g of the three-arm macroinitiator obtained in step 1 in 1 mL of DMSO, and add the solution contain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com