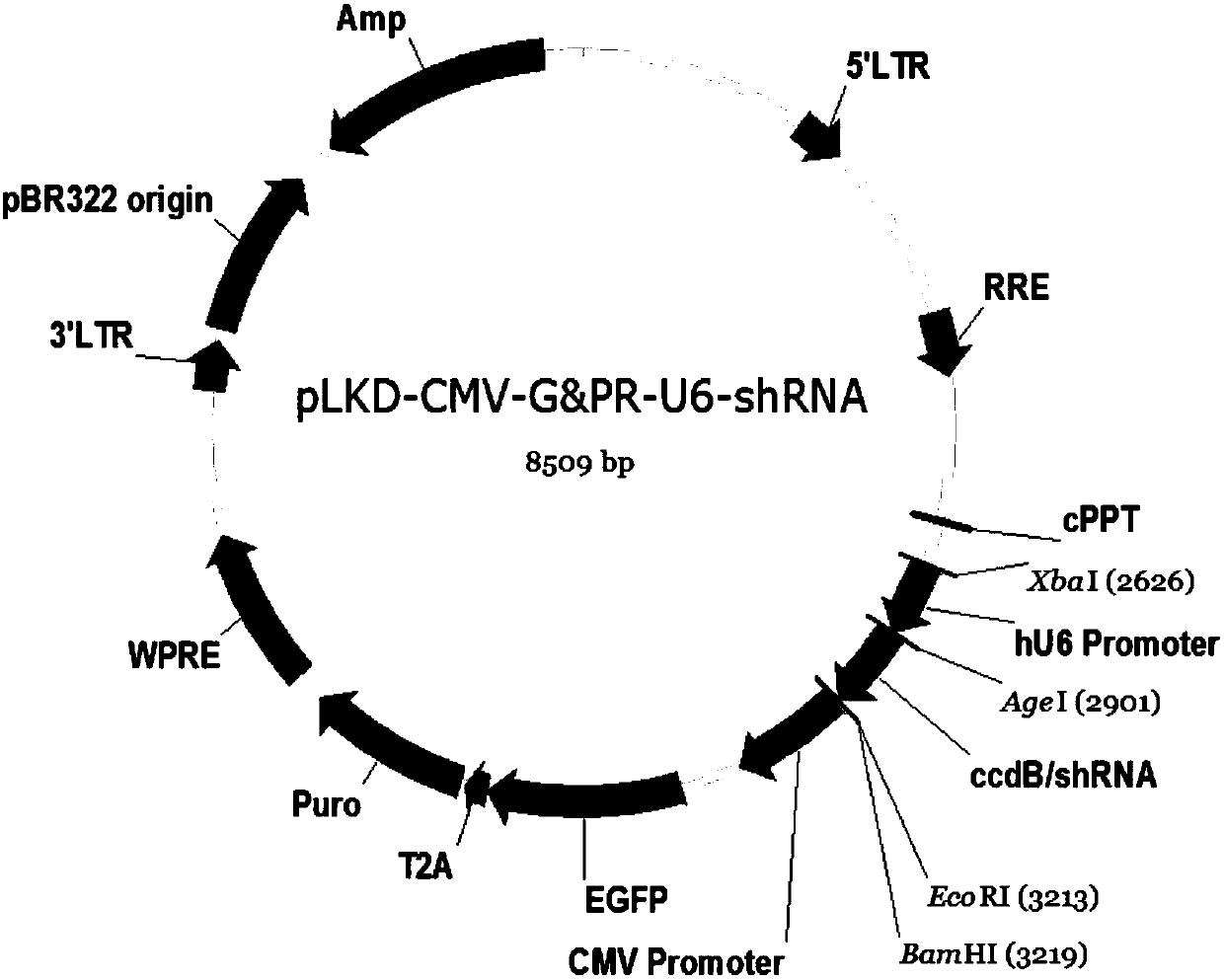
ShRNA sequence for inhibiting replication of influenza A virus and application of shRNA sequence
A type A influenza virus and sequence technology, applied in the direction of DNA / RNA fragments, antiviral agents, recombinant DNA technology, etc., can solve the problems of influenza vaccine failure, evasion of defense, influenza virus human health threats, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Construction of influenza virus NP fragment shRNA expression plasmid, transformation of Escherichia coli and screening of positive clones.
[0039] Influenza A virus, A / Shanghai / N33(2008) / H1N1, virus RNA was extracted, reverse transcribed into cDNA, and Sangon (Shanghai) Biotechnology Co., Ltd. was commissioned to perform DNA sequencing. The sequencing results are as follows:
[0040]
[0041] According to the sequencing results, design the shRNA sequence: GCTGGTCTGACTCACATAATG
[0042] Design viral vector construction framework and DNA primer fragments.
[0043] Table 1 Viral vector construction framework
[0044]
[0045] DNA primer fragment:
[0046]NP-391-F CcggGCTGGTCTGACTCACATAATGCTCGAGCATTATGTGAGTCAGACCAGCTTTTTTg
[0047] NP-391-R aattcaaaaaaGCTGGTCTGACTCACATAATGCTCGAGCATTATGTGAGTCAGACCAGC
[0048] Expression vector: lentiviral vector (pLKD-CMV-G&PR-U6-shRNA). The interference fragment was constructed into the downstream of the U6 promoter of the lent...
Embodiment 2
[0086] Influenza virus interference plasmid pLKD-NP-391 inhibits the replication of influenza virus in MDCK cells.
[0087] 1. Plasmid extraction:
[0088] According to Promega's Wizard Plus Minipreps DNA Purification System kit instructions.
[0089] 2. Plasmid transfection into MDCK cells:
[0090]MDCK cells are the Madin–Darby canine kidney cell line, which is the most commonly used tool cell for studying influenza virus infection. After recovery, MDCK cells were cultured in DMEM medium containing heat-inactivated 10% FCS, 2mM L-glutamine, 100 units of penicillin / ml and 100 μg / ml of streptomycin, after 2-3 passages, inoculated In a 24-well cell culture plate, the seeding density was 5×10 4 / hole. 37°C, 5% CO 2 , cultivated at saturated humidity for 24 hours. When the cell layer reached 60%-70% confluence, the MDCK cells were transfected with the interference plasmid pLKD-NP-391 and the empty plasmid pLKD-007, respectively. For the operation steps, see the instructions...
Embodiment 3
[0106] Influenza virus interference plasmid pLKD-NP-391 inhibits the replication of influenza virus in mice.
[0107] 1. Plasmid extraction: Use the AxyPrep Endotoxin-Free Plasmid Mass Kit of Aisijin Biotechnology (Hangzhou) Co., Ltd. to extract interfering plasmids and empty plasmids. The operating steps were in accordance with the kit instructions provided by the company.
[0108] 2. Experimental animals and grouping: 30 BALB / C mice. Female, 6-8 weeks old. They were randomly divided into control group, empty plasmid group and interference plasmid group, with 10 mice in each group.
[0109] 3. Animals transfected with plasmids: PLKD-NP-391 and PLKD-007 (empty plasmids) were mixed thoroughly with PEI (polyetherimide) at room temperature for 20 minutes at a nitrogen / phosphorus weight ratio of 15:1. Nebulize PEI / PLKD-NP-391 or PEI / PLKD-007 complexes respectively with PARIBOY nebulizer. The aerosol was expelled into a closed plastic box containing 10 mice. Mice in an aerosol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com