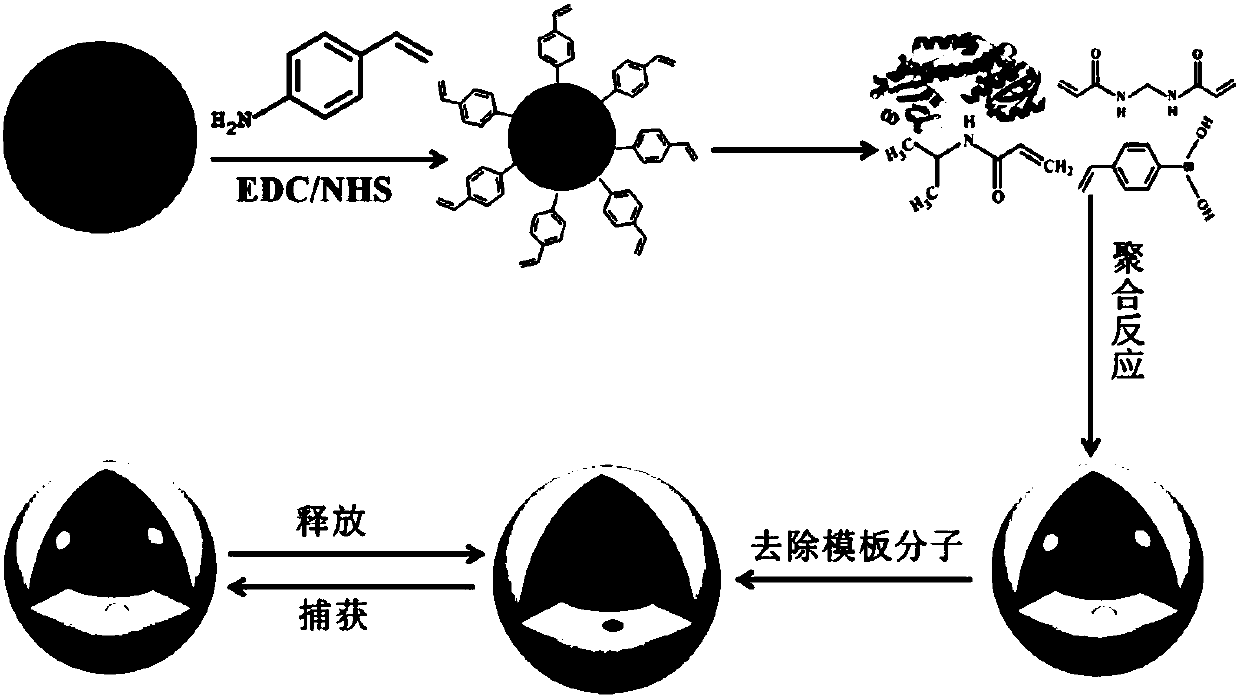
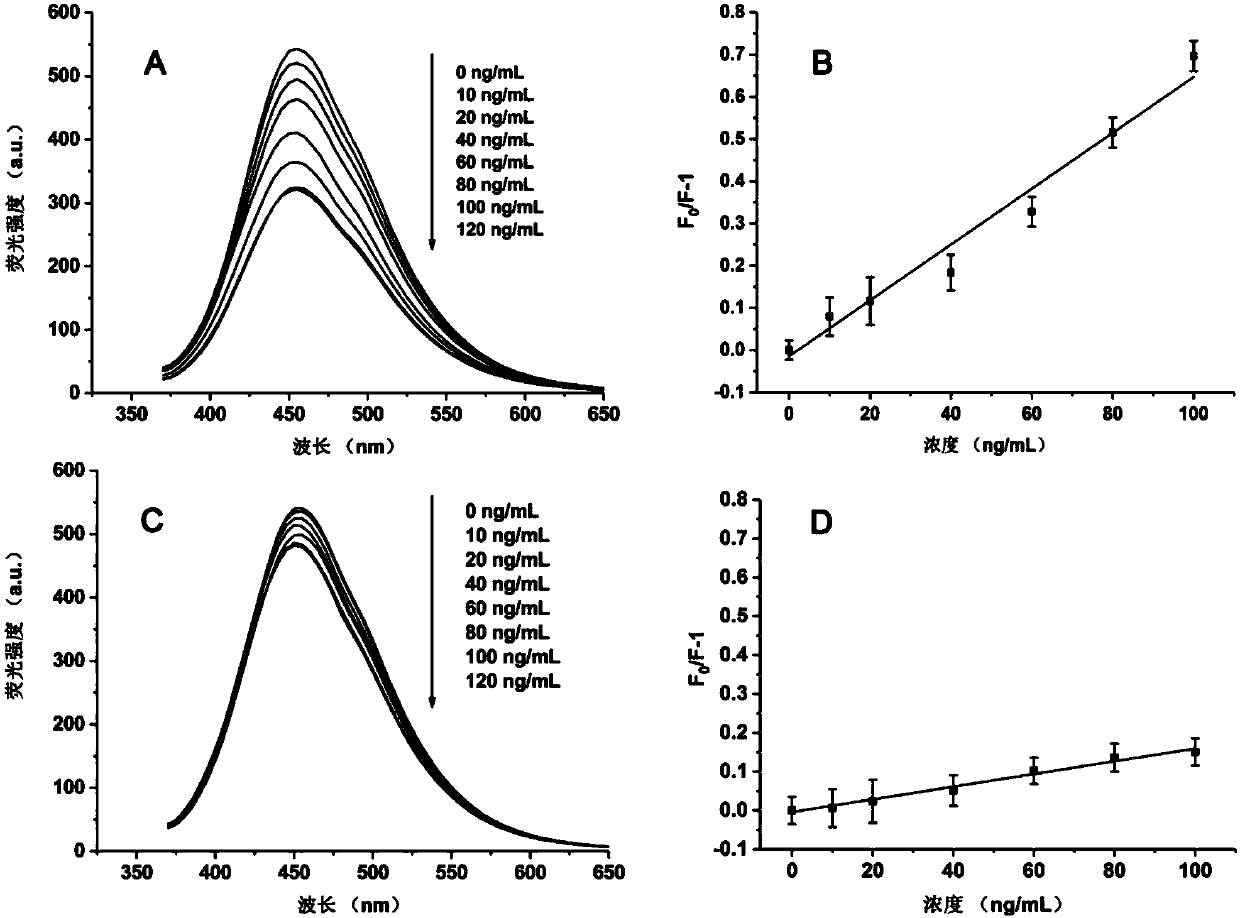
Fluorescent nanometer molecular imprinting biomimetic sensor, preparation method and applications thereof
A technology of molecular imprinting and fluorescent nanometers, applied in fluorescence/phosphorescence, material excitation analysis, etc., can solve the problems of fast, economical, convenient, high-sensitivity and high-specificity detection of alpha-fetoprotein, achieve good molecular recognition ability, shorten Time, accuracy and precision results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Add 1.051 g of citric acid and 335 μL of ethylenediamine into 20 mL of ultrapure water, stir to dissolve; transfer the resulting solution to a 50 mL polytetrafluoroethylene autoclave, and react at 200 ° C for 5 h; naturally cool to room temperature, and pure Dialyzed in water for 48 hours, and freeze-dried for 12 hours to obtain carbon dots for future use.
[0033] (2) Ultrasonic dispersion of 20 mg of carbon dot powder in 20 mL of phosphate buffer solution with pH=5, followed by adding 100 μL of 1M EDC and NHS, and magnetic stirring for 20 minutes; adding 300 μL of 0.25M 4-VPA to the above solution for covalent condensation reaction, Magnetic stirring reaction 4h. After the reaction, the reaction solution was dialyzed in water for 48 hours to remove unreacted 4-VPA, other reactants and ions. Freeze-dry for 12 hours to obtain carbon dots modified with double bonds on the surface, which are ready for use.
[0034] (3) Add 1.25mg / mL AFP 160μL, 0.5mmol NIPAAm and 4-V...
Embodiment 2
[0037] (1) Add 1.051 g of citric acid and 335 μL of ethylenediamine into 20 mL of ultrapure water, stir to dissolve; transfer the resulting solution to a 50 mL polytetrafluoroethylene autoclave, and react at 200 ° C for 5 h; naturally cool to room temperature, and pure Water dialysis for 48 hours, and freeze-drying for 12 hours to obtain carbon dots for future use.
[0038] (2) Ultrasonic dispersion of 20 mg of carbon dot powder in 20 mL of phosphate buffer solution with pH=5, followed by adding 200 μL of 1M EDC and NHS, and magnetic stirring for 20 minutes; adding 300 μL of 0.25M 4-VPA to the above solution for covalent condensation reaction, Magnetic stirring reaction 4h. After the reaction was completed, the reaction solution was dialyzed in water for 48 hours to remove unreacted 4-VPA, other reactants and ions. Freeze-dry for 12 hours to obtain carbon dots modified with double bonds on the surface, which are ready for use.
[0039] (3) Add 1.25mg / mL AFP 320μL, 0.5mmol NI...
Embodiment 3
[0042] (1) Add 1.051 g of citric acid and 335 μL of ethylenediamine into 20 mL of ultrapure water, stir to dissolve; transfer the resulting solution to a 50 mL polytetrafluoroethylene autoclave, and react at 200 ° C for 5 h; naturally cool to room temperature, and pure Water dialysis for 48 hours, and freeze-drying for 12 hours to obtain carbon dots for future use.
[0043] (2) Ultrasonic dispersion of 20mg carbon dot powder in 20mL pH=5 phosphate buffer solution, then add 200μL 1M EDC and NHS in turn, and magnetic stirring reaction for 20min; add 300μL 0.25M 4-VPA to the above solution for covalent condensation reaction, magnetic The reaction was stirred for 4h. After the reaction was completed, the reaction solution was dialyzed in water for 48 hours to remove unreacted 4-VPA, other reactants and ions. Finally, freeze-dry for 12 hours to obtain carbon dots modified with double bonds on the surface, which are ready for use.
[0044] (3) Take 1.25mg / mL AFP 320μL, 0.5mmol NIP...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com