Method for synthesizing m-hydroxybenzaldehyde
A technology of hydroxybenzaldehyde and m-hydroxybenzoyl chloride, which is applied in the field of synthesizing m-hydroxybenzaldehyde, can solve the problems of large amount of waste water and salt, prominent environmental protection problems, and high environmental pressure, and achieve high product yield and equipment occupancy The effect of small size and fewer production steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
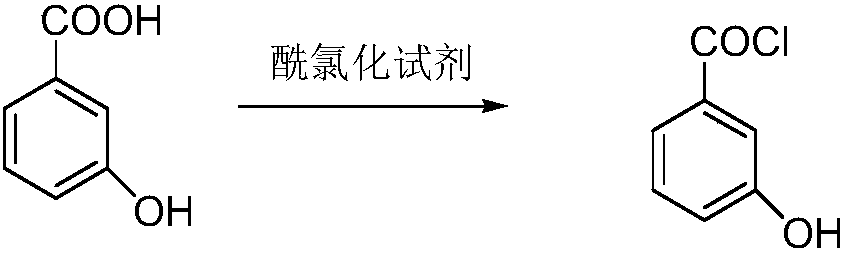
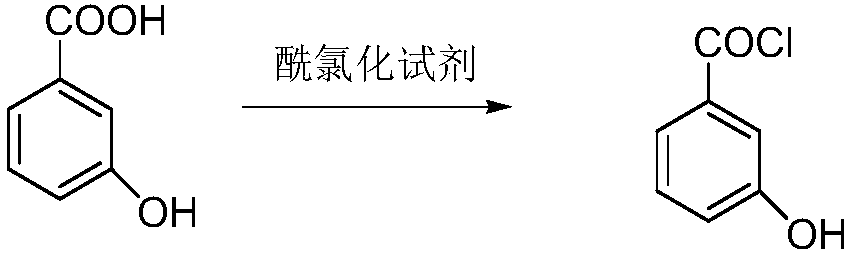
[0029] Step (1) acid chloride:
[0030] Add 100 grams of m-hydroxybenzoic acid, 600 milliliters of toluene and 1 milliliter of N,N-dimethylformamide into the reaction flask, raise the temperature to 110°C, start to add 115 grams of thionyl chloride dropwise, and complete the addition within 1-3 hours. After adding and keeping warm, HPLC monitors the reaction process until there is no m-hydroxybenzoic acid, and the reaction is stopped to obtain m-hydroxybenzoyl chloride solution.
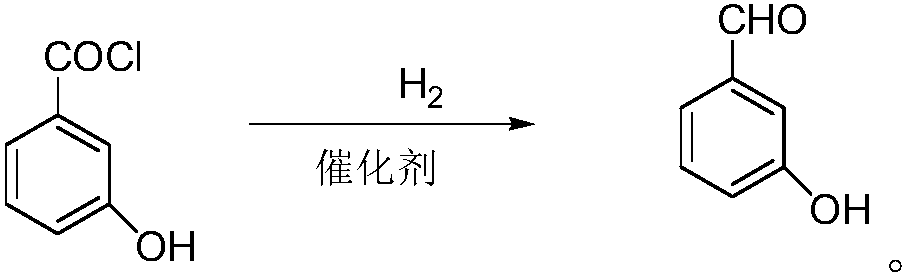
[0031] Step (2) hydrogenation reduction:
[0032] Add the m-hydroxybenzoyl chloride solution obtained in the previous step into the autoclave, add 20 grams of palladium carbon catalyst and 0.01 gram of poisoning agent quinoline / sulfur, add 150 grams of triethylamine, heat up to 120 ° C, and feed hydrogen until the pressure is 0.6MPa, start the reaction, during which hydrogen is constantly replenished to maintain the pressure in the reactor, react until the liquid phase monitors that there is no raw ...
Embodiment 2
[0035] Step (1) acid chloride:
[0036] Add 100 grams of m-hydroxybenzoic acid, 600 milliliters of xylene and 1 milliliter of N,N-dimethylformamide into the reaction flask, raise the temperature to 110°C, start adding 122 grams of oxalyl chloride dropwise, and finish adding in 1-3 hours. Complete heat preservation, HPLC monitors the reaction process until there is no m-hydroxybenzoic acid, stop the reaction, and obtain m-hydroxybenzoyl chloride solution.
[0037] Step (2) hydrogenation reduction:
[0038] Add the m-hydroxybenzoyl chloride solution obtained in the previous step into the autoclave, add 20 grams of palladium / barium sulfate catalyst, add 120 grams of sodium acetate, raise the temperature to 110 ° C, pass hydrogen to the pressure of 0.8 MPa, and start the reaction. Supplement hydrogen to maintain the pressure in the reactor until there is no raw material peak in the liquid phase monitoring, that is, the end of the reaction is reached, the reaction is stopped, the ...
Embodiment 3
[0041] Step (1) acid chloride:
[0042] Add 100 grams of m-hydroxybenzoic acid, 800 milliliters of dichloroethane and 1 milliliter of N,N-dimethylformamide into the reaction flask, raise the temperature to 110°C, and start adding 155 grams of phosphorus oxychloride dropwise for 1-3 hours After the addition is completed, keep warm after the addition, and monitor the reaction process by HPLC until there is no m-hydroxybenzoic acid, then stop the reaction to obtain m-hydroxybenzoyl chloride solution.
[0043] Step (2) hydrogenation reduction:
[0044] Add the m-hydroxybenzoyl chloride solution obtained in the previous step into the autoclave, add 5 grams of palladium / calcium carbonate catalyst, raise the temperature to 80°C, and let the hydrogen flow until the pressure is 0.4MPa to start the reaction. When there is no raw material peak, the end of the reaction is reached, the reaction is stopped, the reaction solution is filtered, and the filtrate is distilled under reduced pressu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com