Hog cholera virus E2 protein epitope and preparation and application of antibody
A technology of swine fever virus and antigenic epitope, which is applied in the direction of antiviral immunoglobulin, viral peptides, material inspection products, etc., can solve the problems of no detailed reports, etc., and achieve high coincidence rate, short production cycle and good specificity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Materials and methods
[0032] The strains, cells and experimental animals of CSFV Shimen strain, SP2 / 0 myeloma cells, PK-15 and Vero cells are all preserved by the Shanghai Veterinary Research Institute of the Chinese Academy of Agricultural Sciences. Cells were kept at 37°C, 5% CO 2 and 10% FBS (FBS, Gibico, Shanghai, China) conditions. BALB / c female mice were purchased from Shanghai Slack Experimental Animal Company.
[0033] Vectors, reagents and strains pCold-TF vector, Escherichia coli BL21 (DE3) were purchased from Takara (Shanghai) Company; HRP-labeled goat anti-mouse IgG and FITC-labeled goat anti-mouse IgG were purchased from Sigma (Shanghai) Company; Enzyme EcoR I was purchased from NEB (Shanghai) Company.
Embodiment 2
[0034] Example 2: Amplification and protein preparation of the main epitope of E2
[0035] Amplification of E2 Main Antigen Epitope Region Gene and Construction of Recombinant Plasmid
[0036] According to the CSFV E2 gene sequence published by NCBI, the 1st-537th nucleotide sequence of the E2 gene was sent to the company (Jinweizhi) for optimization and synthesis, and the synthetic E2 gene was connected with the pCold-TF vector to construct a plasmid. After the plasmid was sequenced correctly , the positive plasmid was named pCold-E2.
[0037] Induced expression and purification of recombinant proteins
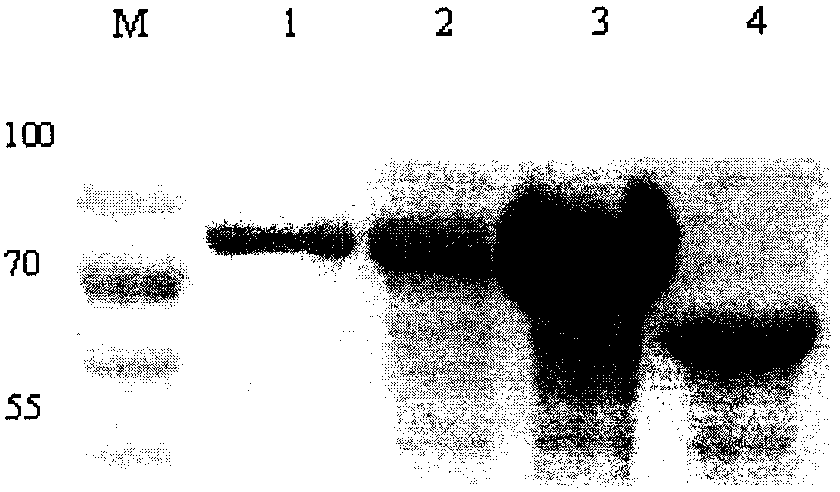
[0038] The recombinant plasmid pCold-E2 was transformed into BL21(DE3) Escherichia coli, cultured with shaking at 37°C until the OD600 value reached 0.6-0.8, then induced by adding IPTG with a final concentration of 1 mM, shaking at 16°C for 24 hours. The bacteria were collected and added to PBS for ultrasonic lysis, added 5×SDS buffer and boiled for 10 min, followed by SDS...
Embodiment 3
[0040] Embodiment 3: Preparation of monoclonal antibody
[0041] mouse immunization
[0042] 100 μg of purified protein was mixed and emulsified with Freund's complete adjuvant 1:1, and inoculated into 6-week-old healthy female BALB / c mice, and then every 2 weeks, the same amount of purified protein was emulsified with Freund's incomplete adjuvant for the second and For the third immunization, blood was collected on the 10th day after the third immunization, and the ELISA antibody titer was determined. For mice with a titer above 1:10000, 200 μg of pure antigen was injected intraperitoneally on the 3-4 day before cell fusion for booster immunity.
[0043] Preparation of McAbs
[0044] One day before fusion, mouse peritoneal macrophages were used as feeder cells, and then the mouse with the highest immune titer was selected for fusion of splenocytes and SP2 / 0 cells. The hybridoma cells were cultured in a 96-well plate lined with feeder cells at 37°C and 5% CO2 under the condit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com