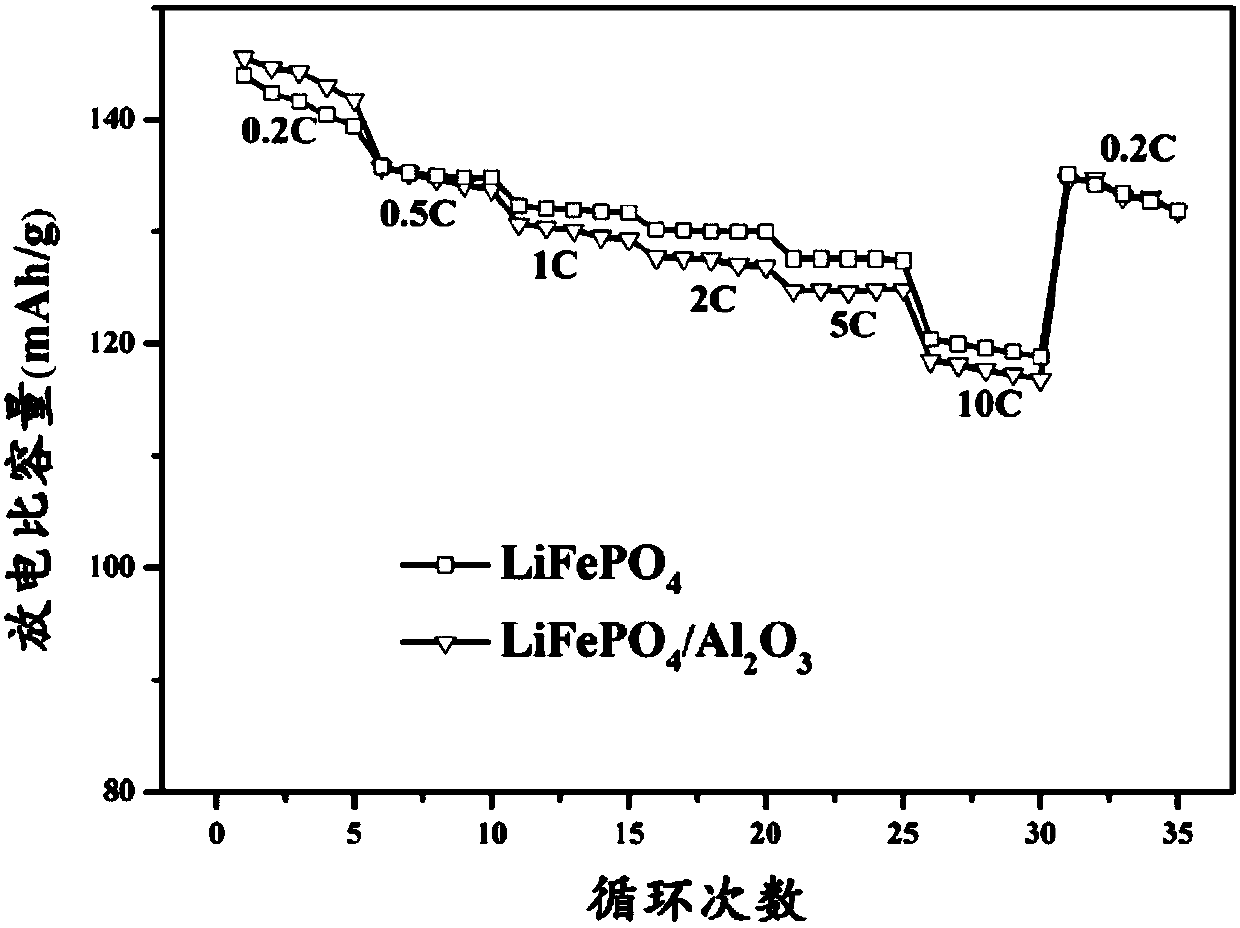
Lithium iron phosphate composite electrode with metal oxide coating layer on surface
A technology of oxide coating and lithium iron phosphate, which is applied in electrode manufacturing, battery electrodes, circuits, etc., can solve problems affecting the structural stability of lithium iron phosphate, increase the difficulty of process processing, and increase the hygroscopicity of materials, so as to achieve heat suppression Effects of runaway reaction, reduction of dissolution, and inhibition of corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Lithium iron phosphate, acetylene black and polyvinylidene fluoride (PVDF) are evenly mixed into a slurry, and the slurry is evenly coated on the aluminum current collector with an automatic coating machine, dried, and rolled to prepare a lithium iron phosphate electrode piece. Among them, the mass ratio of lithium iron phosphate: acetylene black: PVDF is 85:7:8, and the thickness of the lithium iron phosphate pole piece after rolling is about 55 μm (collector fluid).
[0027] Add 10g of Al with particle size D50=300nm in 100g of deionized water 2 o 3 Granules and 1g polyvinylpyrrolidone (PVP), after mixing evenly, use a planetary ball mill to stir and disperse for 2 hours at a speed of 400rpm, then add 12g of 50% solid content of styrene-butadiene rubber (SBR) and 1.5g of sodium carboxymethylcellulose (CMC) (actual binder content 7.5g), continue to adopt planetary ball mill to continue to disperse 12h with the rotating speed of 400rpm, obtain the evenly dispersed Al ...
Embodiment 2
[0034] Add 15g of SiO with particle size D50=50nm in 150g deionized water 2 Granules and 1g of polyethylene glycol (PEG), mixed evenly, stirred and dispersed with a planetary ball mill at a speed of 400rpm for 24h, then added 60g of sodium alginate aqueous solution (sodium alginate: water = 1:5wt%) (actual binder content 10g), continue to use the planetary ball mill to stir and disperse at a speed of 600rpm for 12h to obtain evenly dispersed SiO 2 slurry. The resulting slurry was coated on the surface of the lithium iron phosphate pole piece by spin coating. The spin coating time was 180s, and the thickness of the deposited layer was about 3 μm. After natural drying at 60°C, it was dried in vacuum at 120°C for 12h to obtain the surface SiO 2 Coated lithium iron phosphate composite electrode, the thickness of the pole piece is increased by about 1 μm, SiO 2 It accounts for about 0.5% of the pole piece weight.
Embodiment 3
[0036] Add 15g of Al with particle diameter D50=500nm in 100g of ethanol 2 o 3 Particles and 1g of PVP, after mixing evenly, use a planetary ball mill to stir and disperse at 200rpm for 2 hours, then add 55g of polyvinylidene fluoride (PVDF) in acetone solution (PVDF: acetone = 1:10wt%) (the actual binder quality is 5g), Continue to use the planetary ball mill to stir and disperse at a speed of 300rpm for 12h to obtain evenly dispersed Al 2 o 3 slurry. After the obtained slurry was heated to 40°C, it was coated on the surface of the lithium iron phosphate pole piece by the dip coating method. During the dip coating, the dip coating thickness of the electrode was controlled to be 120 μm (the thickness of the pole piece increased by about 9.2 μm after dip coating and drying, and the quality increased. about 4.7%), after natural drying at 60°C, vacuum drying at 120°C for 12 hours to obtain surface Al 2 o 3 Coated lithium iron phosphate composite electrode.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com