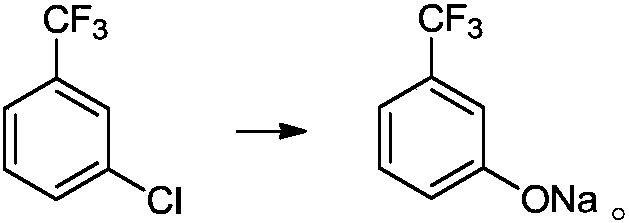
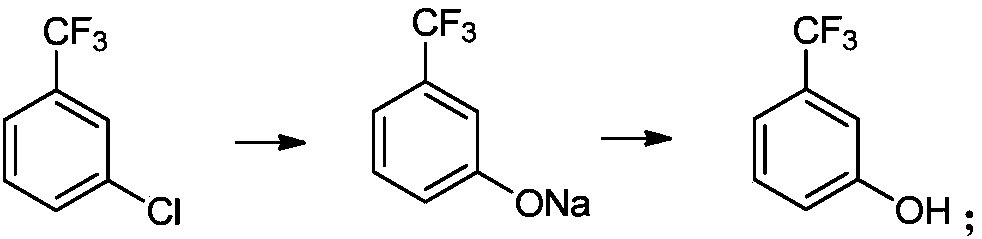
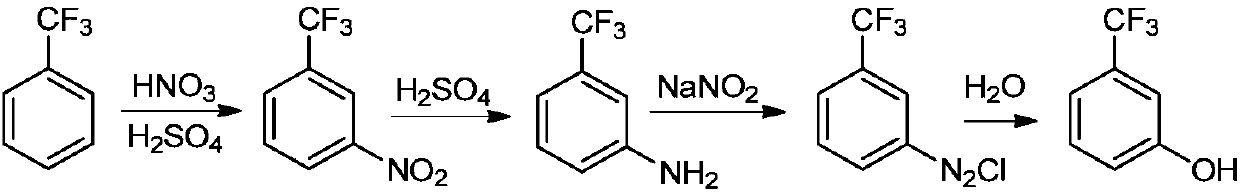
Preparation method of m-trifluoromethylphenol
A technology of sodium trifluoromethyl phenate and trifluoromethyl chlorobenzene, which is applied in the field of m-trifluoromethyl phenol preparation, can solve the problems of large environmental pollution, high equipment requirements, and high safety risks, and achieve low environmental pollution, High product yield and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 1L autoclave, add 180.5g (1.0mol) m-trifluoromethylchlorobenzene, add 361g methanol, 80g (2.0mol) sodium hydroxide solid, 7.0g (0.02mol) 8-hydroxyquinoline copper, after adding, Close the kettle and heat up to 100~105℃, keep the kettle pressure at 0.8~1.0MPa, keep the temperature of the reaction system between 100~105℃, react for 5h, observe that the kettle pressure reaches 1.5~1.7MPa, centrally control sampling, check that the raw materials have reacted , M-trifluoromethyl phenol sodium HPLC=96.7%; the temperature of the reaction kettle is lowered, the reaction liquid is transferred to the normal pressure kettle, and the temperature of the kettle is raised to 35~40℃ under the vacuum degree of -0.03MPa, and the methanol is evaporated and recycled . After the steaming is completed, add 150 g of water to the system, adjust the pH of the reaction solution to between 7 and 8 with 36% hydrochloric acid, filter, and recover the catalyst. The mother liquor was filtered with 36...
Embodiment 2
[0054] 1L autoclave, add 180.5g (1.0mol) m-trifluoromethylchlorobenzene, add 361g methanol, 112g (2.0mol) potassium hydroxide solid, 7.3g (0.02mol) 4-methyl-8-hydroxyquinoline copper After the addition is complete, close the kettle and heat up to 100~105℃, keep the kettle pressure at 0.8~1.0MPa, keep the temperature of the reaction system between 100~105℃, react for 4h, observe that the kettle pressure reaches 1.5~1.7MPa, control sampling , The detection of raw material reaction is completed, m-trifluoromethyl phenol sodium HPLC = 94.5%; the temperature of the reaction kettle is lowered, the reaction liquid is transferred to the atmospheric kettle, and the temperature of the kettle is raised to 35-40°C under a vacuum of -0.03MPa, Distilled methanol is recycled and reused. After the steaming is completed, add 150 g of water to the system, adjust the pH of the reaction solution to between 7 and 8 with 36% hydrochloric acid, filter, and recover the catalyst. The mother liquor was...
Embodiment 3
[0056] 1L autoclave, add 180.5g (1.0mol) m-trifluoromethylchlorobenzene, add 361g n-propanol, 100g (2.5mol) sodium hydroxide solid, 5.2g (0.02mol) copper acetylacetonate, after adding, close The kettle is heated to 100~110℃, keep the kettle pressure at 0.8~1.0MPa, keep the temperature of the reaction system between 100~110℃, react for 6h, observe that the kettle pressure reaches 1.5~1.7MPa, centrally control sampling, check the raw material reaction is completed, Sodium m-trifluoromethylphenol HPLC=95.9%; the temperature of the reaction kettle is lowered, the reaction liquid is transferred to the atmospheric kettle, and the temperature of the kettle is raised to 35~40℃ under the vacuum degree of -0.03MPa, and the n-propanol is evaporated and recovered Apply. After the steaming is completed, add 150 g of water to the system, adjust the pH of the reaction solution to between 7 and 8 with 36% hydrochloric acid, filter, and recover the catalyst. The mother liquor was filtered with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com