Immobilized ionic liquid catalyst, and preparation method and application thereof
An ionic liquid and catalyst technology, applied in chemical instruments and methods, preparation of organic compounds, catalysts for physical/chemical processes, etc., can solve problems such as poor stability and difficult catalyst separation, and achieve favorable dispersion, improved catalytic efficiency and recycling. efficiency, and the preparation method is simple and easy to implement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
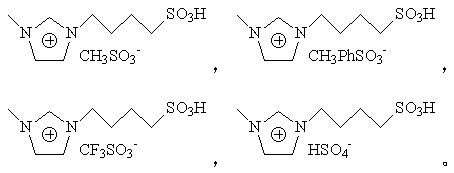
[0033] Weigh 0.98g SBA-16, 0.5g 1-sulfonic acid butyl-3-methylimidazolium methanesulfonate, add to a single-necked flask containing 35ml methanol, N 2 The reaction was refluxed at 68° C. for 24 h under atmosphere. Remove solvent methanol by rotary evaporation, then add 4mmol octyltrimethoxysilane and 10ml dichloromethane, N 2The reaction was carried out at 40°C for 24h under the atmosphere. The reaction product was washed and vacuum-dried to obtain catalyst a.
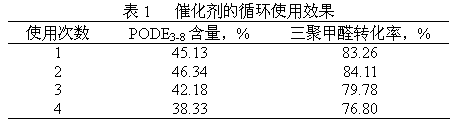
[0034] Add 12.1448g of paraformaldehyde, 6.7303g of methanol, 0.4788g of catalyst a in a 100ml autoclave, and fill with N 2 To the pressure of 1.8MPa, slowly heated to 95 ° C, stirred for 1h. The reaction product was cooled to room temperature, and analyzed by gas chromatography for PODE 3-8 The relative percentage content is 30.48%, and the conversion rate of paraformaldehyde is 64.43%.
Embodiment 2
[0036] Weigh 1.3g SBA-16, 0.64g 1-sulfonic acid butyl-3-methylimidazolium p-toluenesulfonate, add to a single-necked flask containing 30ml methanol, N 2 The reaction was refluxed at 65° C. for 24 h under atmosphere. Rotary evaporation removes solvent methanol, then adds 5mmol dodecyltrimethoxysilane and 15ml dichloromethane, N 2 The reaction was carried out at 40°C for 24h under the atmosphere. The reaction product was washed and vacuum-dried to obtain catalyst b.
[0037] Add 11.8807g of paraformaldehyde, 6.3344g of methanol, 0.5367g of catalyst b in a 100ml autoclave, and fill with N 2 To the pressure of 1.7MPa, slowly heated to 100 ° C, stirring the reaction for 1h. The reaction product was cooled to room temperature, and analyzed by gas chromatography for PODE 3-8 The relative percentage content is 44.62%, and the conversion rate of paraformaldehyde is 82.18%.
Embodiment 3
[0039] Weigh 1.64g SBA-16, 0.71g 1-sulfonic acid butyl-3-methylimidazolium trifluoromethanesulfonate, add to a single-necked flask containing 30ml methanol, N 2 The reaction was refluxed at 65° C. for 24 h under atmosphere. Remove solvent methanol by rotary evaporation, then add 5mmol octyltrimethoxysilane and 15ml dichloromethane, N 2 The reaction was carried out at 40°C for 24h under the atmosphere. The reaction product was washed and vacuum-dried to obtain catalyst c.
[0040] Add 8.9220g of paraformaldehyde, 6.3344g of methanol, 0.4973g of catalyst c in sequence in a 100ml autoclave, and fill with N 2 To the pressure of 1.9MPa, slowly heated to 90 ° C, stirring the reaction for 0.75h. The reaction product was cooled to room temperature, and analyzed by gas chromatography for PODE 3-8 The relative percentage content is 49.95%, and the conversion rate of paraformaldehyde is 85.85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com