pH-responsive fluorescence sensing material based on rhodamine B and cyanobiphenol, preparation method and applications thereof
A cyanobiphenol and fluorescent sensing technology, which is applied in the field of biochemical fluorescent sensing materials, achieves the effects of easy control of conditions, mild conditions, and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
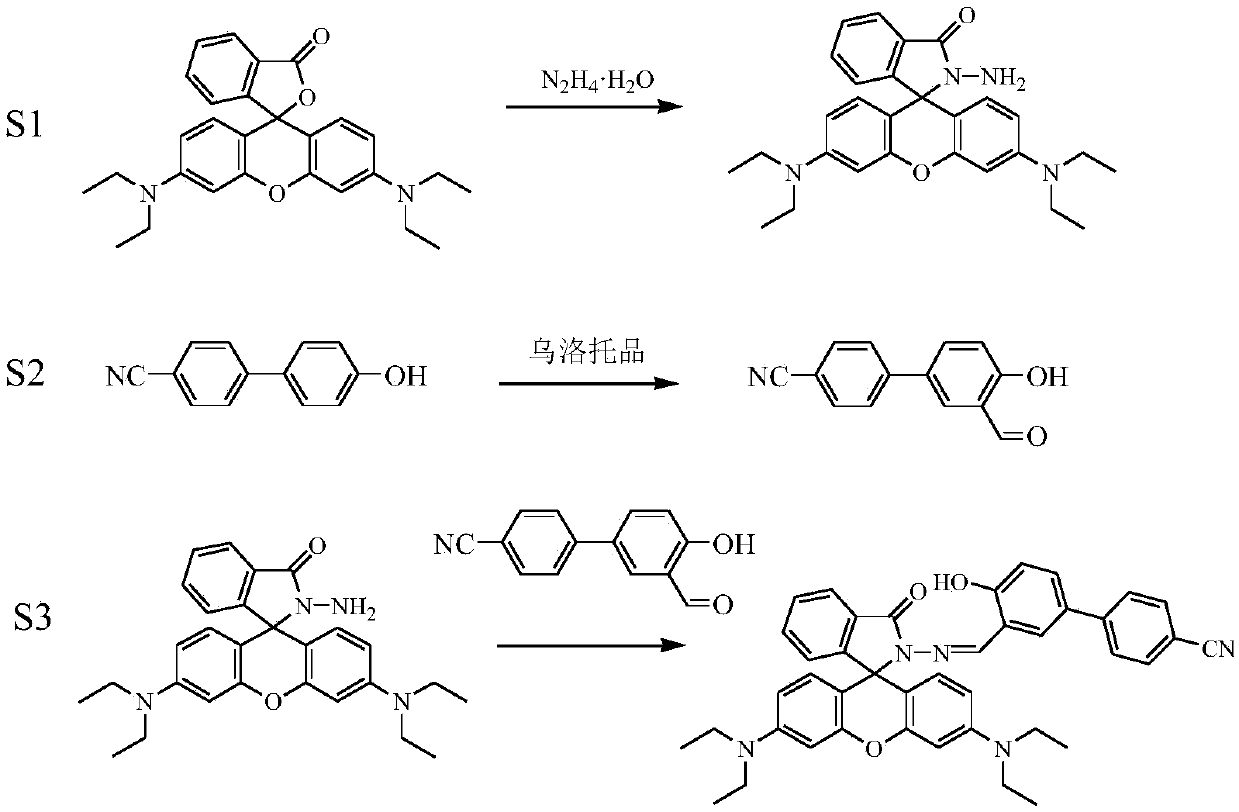
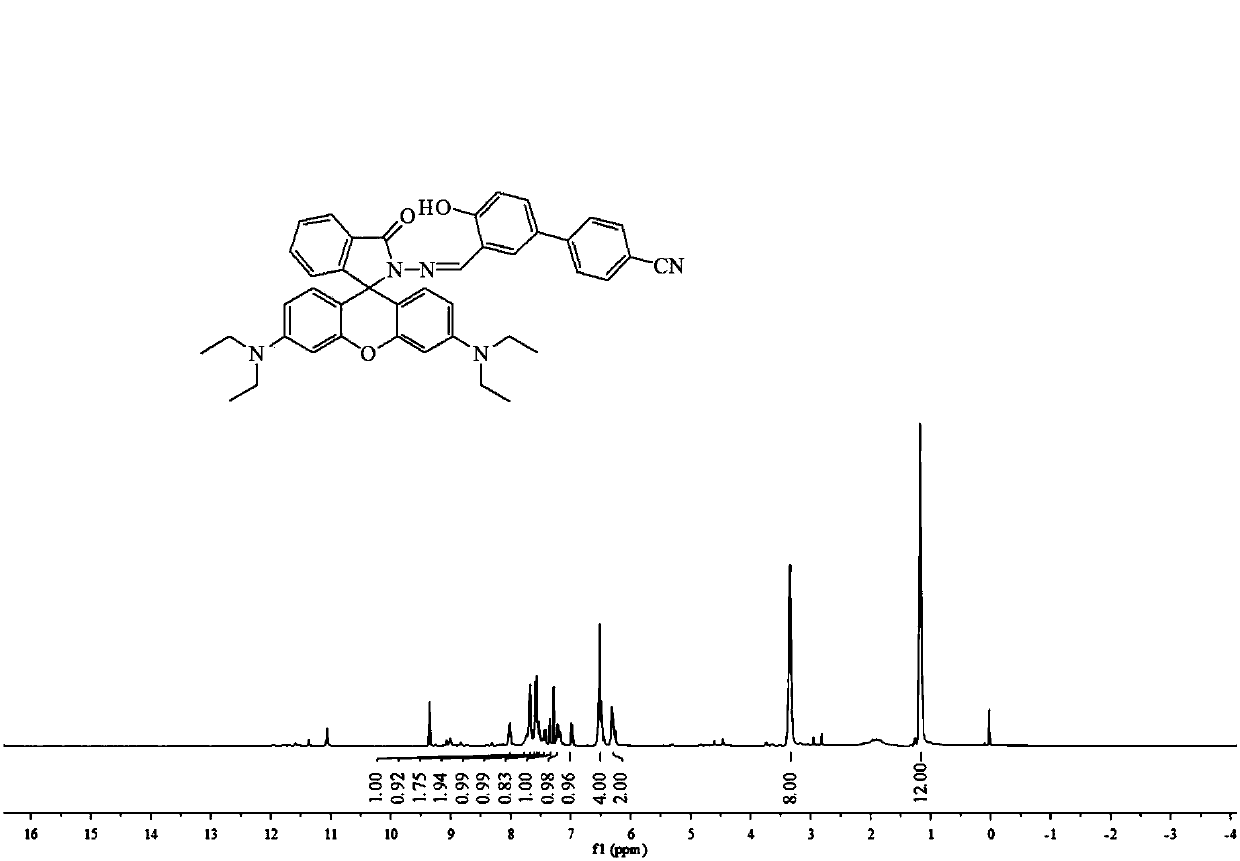
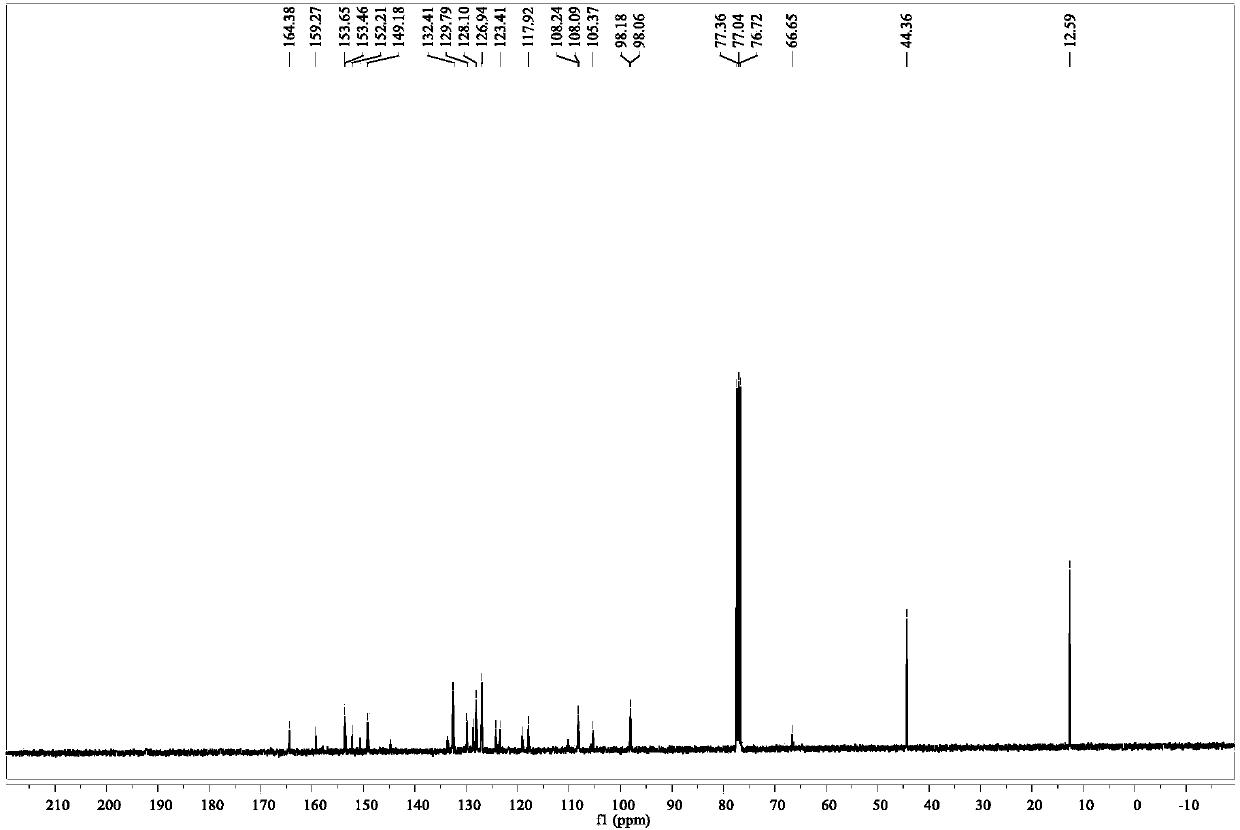
[0034] S1. Synthesis of rhodamine B hydrazide: Dissolve 1 g (2 mmol) rhodamine B in 20 mL of methanol, add 2 mL of 85% hydrazine hydrate dropwise while stirring, and place in a 60°C oil bath after the addition is complete Heat and stir to reflux for 8h. After the reaction is completed, cool to room temperature and remove the solvent under reduced pressure to obtain a crude product, which is purified by recrystallization in methanol.
[0035] S2. Preparation of 3-formyl-4-hydroxybiphenylcyanide: 0.39g (2mmol) of p-cyanobiphenol and 1.41g (10mmol) of urotropine were completely dissolved in 30 mL of trifluoroacetic acid and placed in a round bottom In the flask, heat and stir in an oil bath at 100°C to reflux for 4 hours. After the reaction is complete, cool the system to room temperature, add 100 mL of 1.0 M dilute HCl to the system for acidification, then extract the product with dichloromethane, collect the dichloromethane phase and wash it three times with distilled water, an...
Embodiment 2
[0038] S1. Synthesis of Rhodamine B Hydrazide: Dissolve 1.5g (3mmol) Rhodamine B completely in 30mL of methanol, add 3.7 mL of 85% hydrazine hydrate dropwise while stirring, and place in a 65°C oil bath after the addition is complete Heating in medium temperature and stirring to reflux for 10 h, after the reaction was completed, cooled to room temperature, and removed the solvent under reduced pressure to obtain a crude product, which was purified by recrystallization in methanol.
[0039] S2. Preparation of 3-formyl-4-hydroxybiphenylcyanide: 0.78g (4mmol) of p-cyanobiphenol and 2.82g (20mmol) of urotropine were completely dissolved in 50 mL of trifluoroacetic acid and placed in a round bottom In the flask, heat, stir and reflux in an oil bath at 110°C for 6h. After the reaction is complete, cool the system to room temperature, add 100 mL of 1.0 M dilute HCl to the system for acidification, then extract the product with dichloromethane, collect the dichloromethane phase and wa...
Embodiment 3
[0042] S1. Synthesis of Rhodamine B Hydrazide: Dissolve 2g (4mmol) Rhodamine B in 40mL of methanol, add 5 mL of 85% hydrazine hydrate dropwise while stirring, and place in a 70°C oil bath after the addition is complete Heat and stir to reflux for 12 hours. After the reaction is complete, cool to room temperature and remove the solvent under reduced pressure to obtain a crude product, which is purified by recrystallization in methanol.
[0043] S2. Preparation of 3-formyl-4-hydroxybiphenylcyanide: 1.17 g (6 mmol) p-cyanobiphenol and 4.23 g (30 mmol) urotropine were completely dissolved in 70 mL trifluoroacetic acid and placed in a round bottom In the flask, heat, stir and reflux in an oil bath at 120°C for 8 hours. After the reaction is complete, cool the system to room temperature, add 100 mL of 1.0 M dilute HCl to the system for acidification, then extract the product with dichloromethane, collect the dichloromethane phase and wash it three times with distilled water, and dry...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com