Application of theaflavin-3,3'-digallate in preparation of anti-inflammatory medicines
A technology of double gallate, anti-inflammatory drug, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
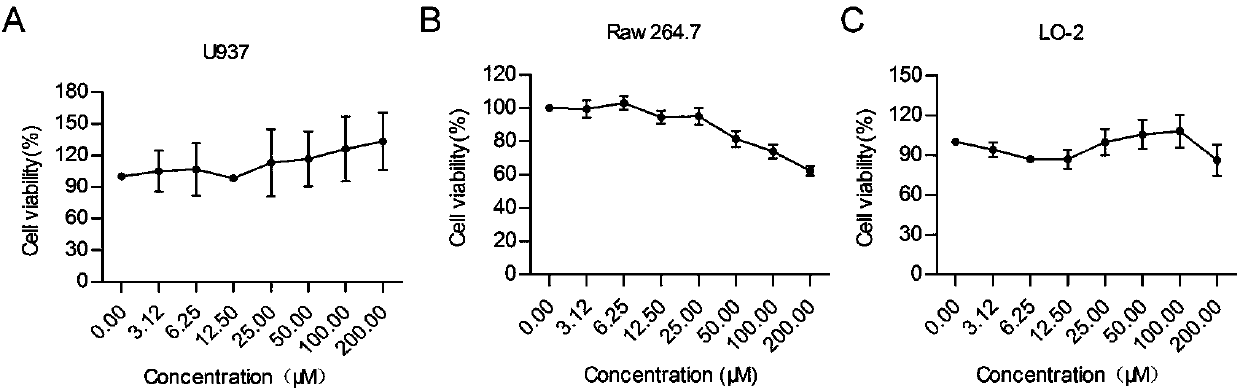
[0023] Example 1 MTT method detects the toxicity of TF-3 to cells
[0024] The specific steps of using the MTT method to detect the toxicity of TF-3 to cells are:
[0025] (1) RAW 264.7, U937, LO-2 cell plating: Evenly spread 100 μL of RAW264.7, U937, LO-2 cells in the logarithmic growth phase in a 96-well plate, and then put it in 37°C, 5% CO 2 Cultivate in a constant temperature incubator until the cell density reaches 80-90%, discard the medium, wash the cells once with PBS buffer, and discard the PBS solution.
[0026] (2) Drug gradient dilution: the drug mother solution was diluted with serum-free DMEM medium into drug working solutions of different concentrations, and the concentrations were 200, 100, 50, 25, 12.5, 6.25, and 3.125 μM in sequence.
[0027] (3) Dosing treatment: 100 μL TF-3 dilution was added to each well, and three replicate wells were set for each drug concentration, and a blank control group, a normal cell control group and a solvent control group were...
Embodiment 2
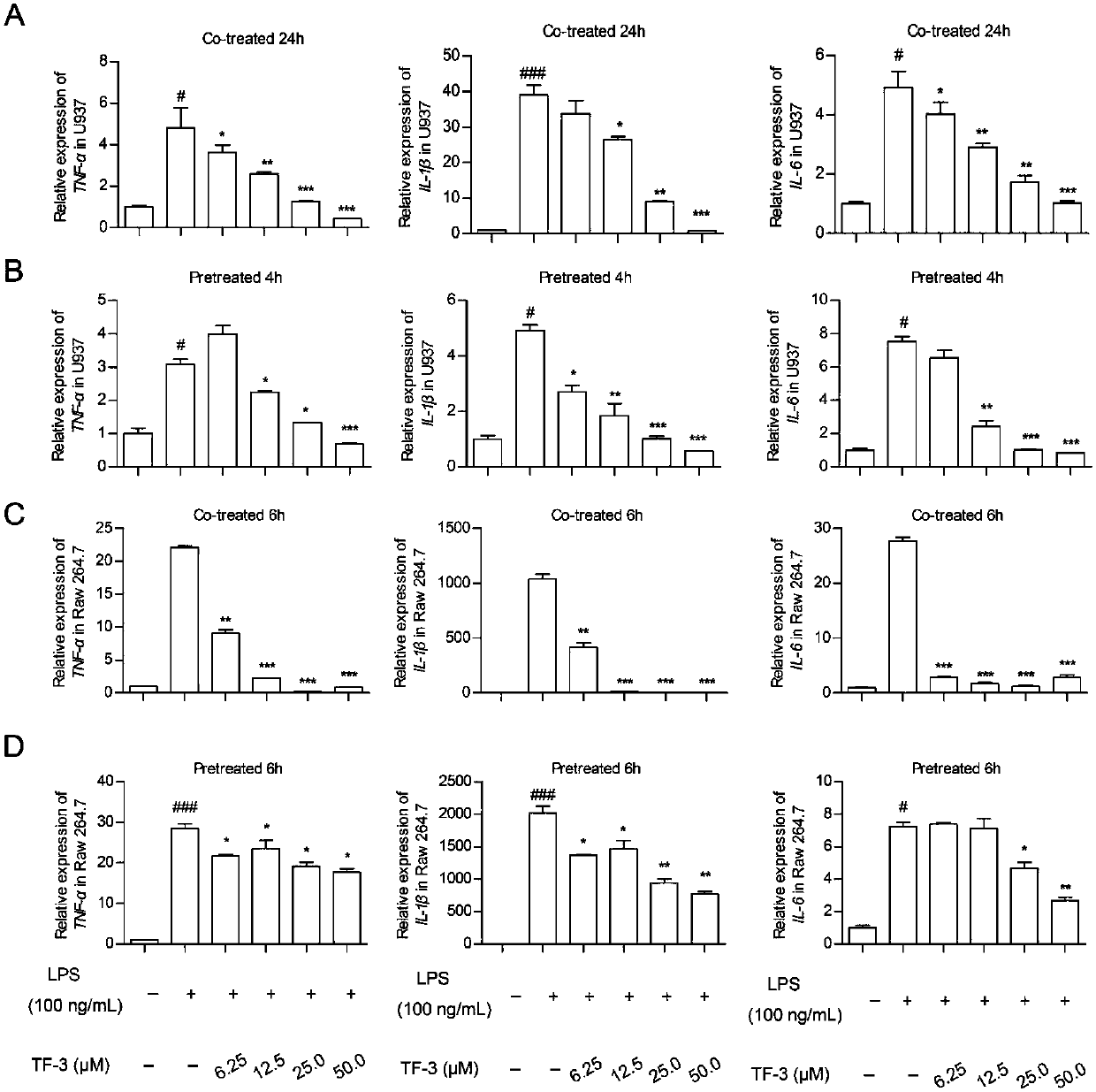
[0032] Embodiment 2 establishes the application model of medicine described in the present invention
[0033] (1) RAW 264.7 cell inflammatory response model
[0034] (1) Dilute the cell suspension to 2×10 5 Cells / mL density, add 1 mL per well into a sterile 12-well plate, shake gently to ensure that the cells are evenly attached to the wall.
[0035] (2) Use serum-free DMEM medium to dilute the 1 mg / mL LPS stock solution to a working solution with a final concentration of 100 ng / mL.
[0036] (3) After culturing overnight, observe the state and confluence of the cells. When the state is good and the density reaches 80-90%, discard the medium, set a normal cell control group without LPS, add serum-free medium, and add to the remaining wells containing Serum-free DMEM medium with 100ng / ml LPS and different concentrations of drugs was placed in a cell culture incubator to continue culturing.
[0037] (4) After 6 hours, total cellular RNA was extracted, reverse-transcribed into ...
Embodiment 3
[0043] Example 3 Detection of inflammatory gene expression level
[0044] (1) Extraction of total RNA from cells
[0045] (1) Pre-preparation: Put on the lab coat, mask and gloves; pre-cool the high-speed refrigerated centrifuge for 10 minutes to lower the temperature to 4°C; prepare 1000 μL, 200 μL, and 10 μL pipette tips without RNase; get ready Chloroform, isopropanol, and reconstituted 75% ethanol.
[0046] (2) Cell lysis: Wash the drug-treated cells 3 times with PBS solution, discard the PBS solution, immediately add 1mL Trizol reagent, place at room temperature for 5min, and repeatedly blow vigorously with a 1mL pipette to fully lyse the cells. Transfer to a 1.5mL EP tube without RNase.
[0047] (3) Phase separation: add 200 μL of chloroform, oscillate on a vortex shaker or vigorously invert to mix, and stand at room temperature for 5 minutes. Centrifuge at 12000g for 15min at 4°C.
[0048] (4) RNA precipitation: Carefully pipette 300-500 μL supernatant (do not suck ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ic50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com