A gynostemma flavonoid compound and its preparation and application in antitumor drugs
A technology of anti-tumor drugs and blue flavonoids, which is applied in the direction of anti-tumor drugs, preparation of sugar derivatives, drug combinations, etc., to achieve good anti-tumor effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Extraction and separation of flavonoids kaempferol-3-O-[2'-trans-coumaroyl-3'-O-β-D-glucosyl-3'-O-β-D from Gynostemma pentaphyllum - Glucorutin:
[0048] (1) Dry the whole herb of Gynostemma pentaphylla (50°C), pulverize it (10 seconds each time, 3-5 times in a row), pass through a 20-mesh sieve to obtain Gynostemma pentaphylla coarse powder; weigh 5Kg Gynostemma pentaphyllum coarse powder, and extract in batches , 500g each time, heat reflux extraction with 75% ethanol aqueous solution, wherein the solid-to-liquid ratio is 1g:15mL, the reflux extraction temperature is 80°C, the reflux extraction time is 2h, and the reflux extraction times are 3 times; then the extracts are combined , the extract was centrifuged (4000r / min, 10min), and the supernatant was concentrated under reduced pressure at 0.095MPa to a paste (40°C) to obtain an ethanol extract;
[0049] (2) Dissolve the ethanol extract in distilled water, extract with petroleum ether (petroleum ether:dis...
Embodiment 2
[0056] Example 2 Determine the structure of kaempferol-3-O-[2'-trans-coumaroyl-3'-O-β-D-glucosyl-3'-O-β-D-glucorutin
[0057] Weigh 15 mg of Compound 1 (trace amount) prepared in Example 1 and dissolve it in a nuclear magnetic resonance tube with deuterated methanol. Using a Bruker DRX-400 nuclear magnetic resonance instrument, use tetramethylsilane (TMS) as an internal standard to measure its hydrogen Spectrum ( 1 H-NMR)( figure 1 ), fully decoupled carbon spectrum ( 13 C-NMR)( figure 2 ). figure 1 Kaempferol-3-O-[2'-trans-coumaroyl-3'-O-β-D-glucosyl-3'-O-β-D-glucorutin 1 H-NMR spectrum; figure 2 Kaempferol-3-O-[2'-trans-coumaroyl-3'-O-β-D-glucosyl-3'-O-β-D-glucorutin 13 C-NMR spectrum.
[0058] Compound 1 is a yellow pine needle-like crystal, developed by polyamide thin-layer chromatography (methanol: water volume ratio is 1:1), showing a single spot; it has yellow fluorescence under 365nm ultraviolet light; spraying 1% aluminum nitrate color developer, Yellow fluo...
Embodiment 3
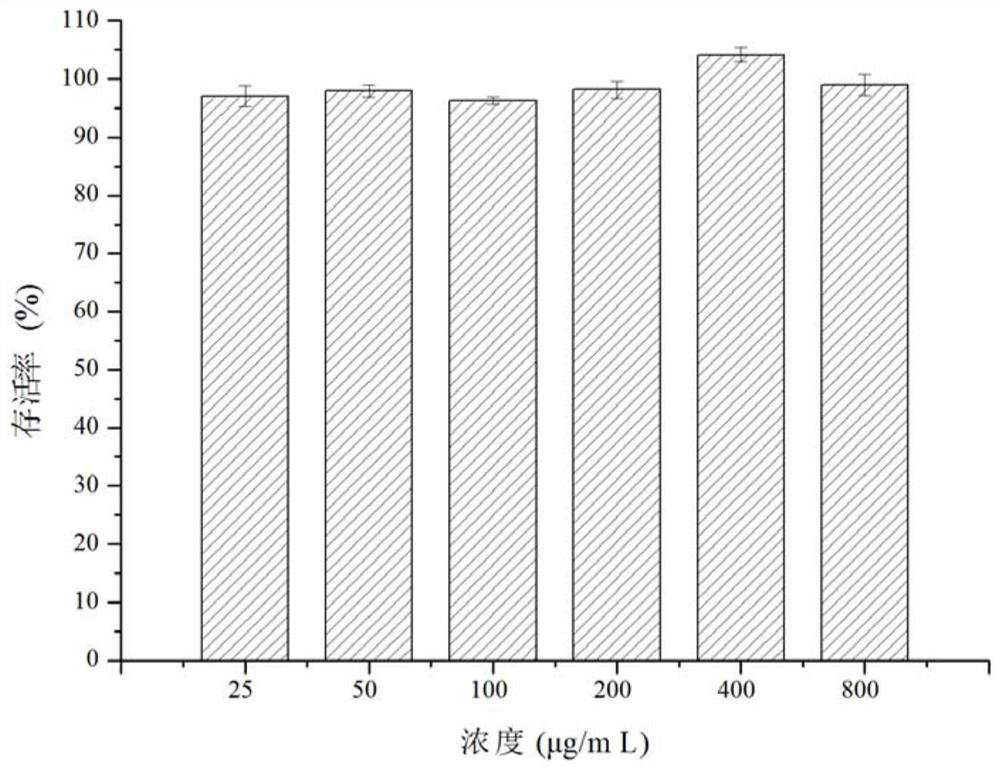
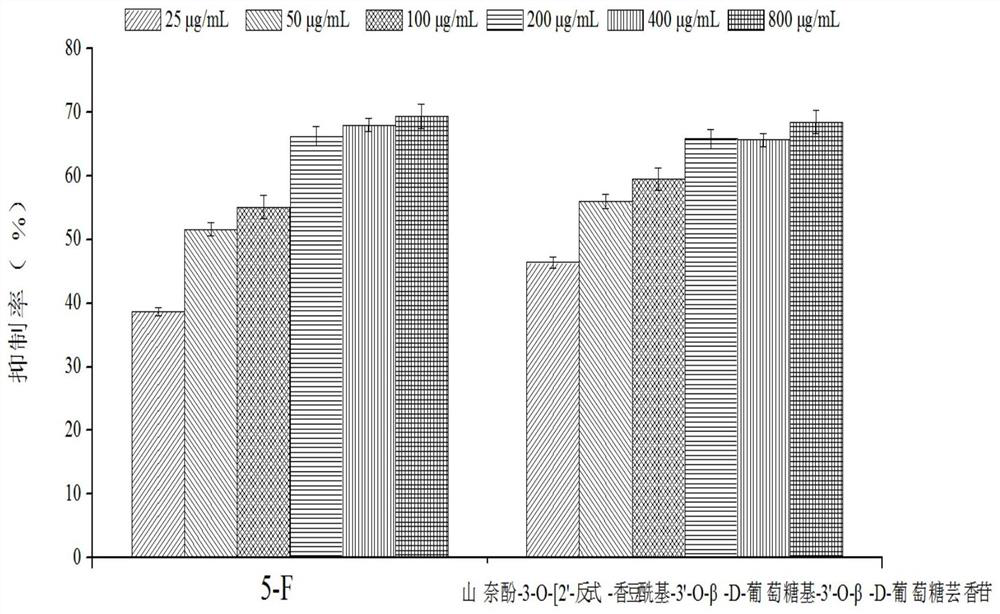
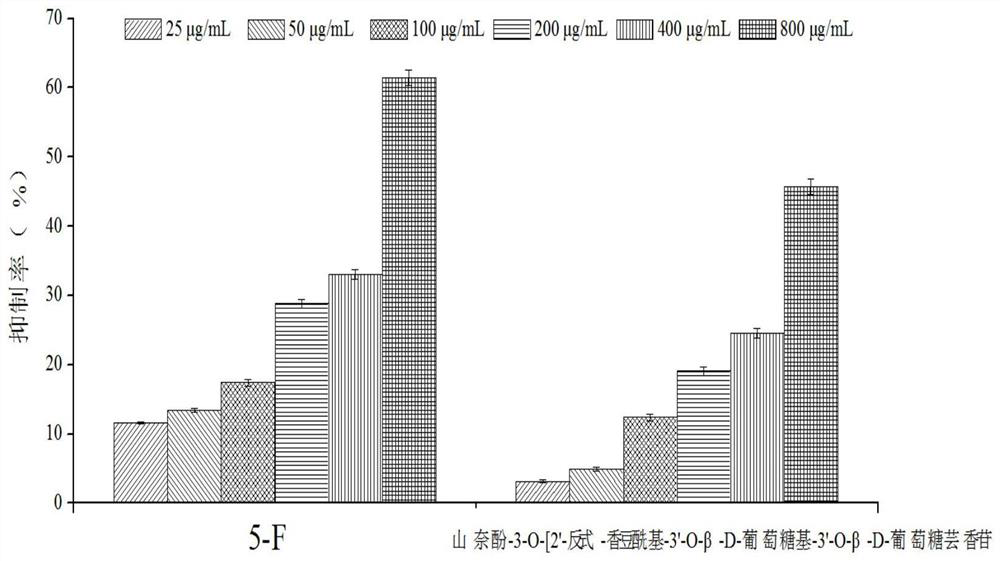
[0068]Example 3 MTT method to detect the effect of kaempferol-3-O-[2'-trans-coumaroyl-3'-O-β-D-glucosyl-3'-O-β-D-glucorutin on human Normal hepatocytes (LO 2 ) Metabolic activity
[0069] Human normal hepatocytes (LO 2 ) to 1×10 5 Each / ml concentration was inoculated in 96-well cell culture plate (100 μL / well), and cultured in a 5% carbon dioxide incubator at 37°C. After the cells in the well plate were completely adhered to the wall, 100 μl of the flavonoid compound solutions prepared in Example 1 at different concentrations (25, 50, 100, 200, 400, 800 μg / mL) were added. At the same time, a positive control group was set up. The positive control sample was 5-fluorouracil (5-Fu), and the same 6 concentration gradients were set. A blank cell control group was also set up in the experiment, that is, only 100 μl of complete culture medium was added, and 6 parallels were set for each concentration. . at 37°C, 5% CO 2 Continue culturing in the incubator, take it out after 24 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com