Method for detecting residue of 18 illegally-added blood-glucose-reducing and antihypertensive drugs in health product
A technology for illegal addition, lowering blood sugar and lowering blood pressure, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of unspecified testing methods and health impacts, and achieve the goal of reducing requirements, shortening time, and improving detection efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
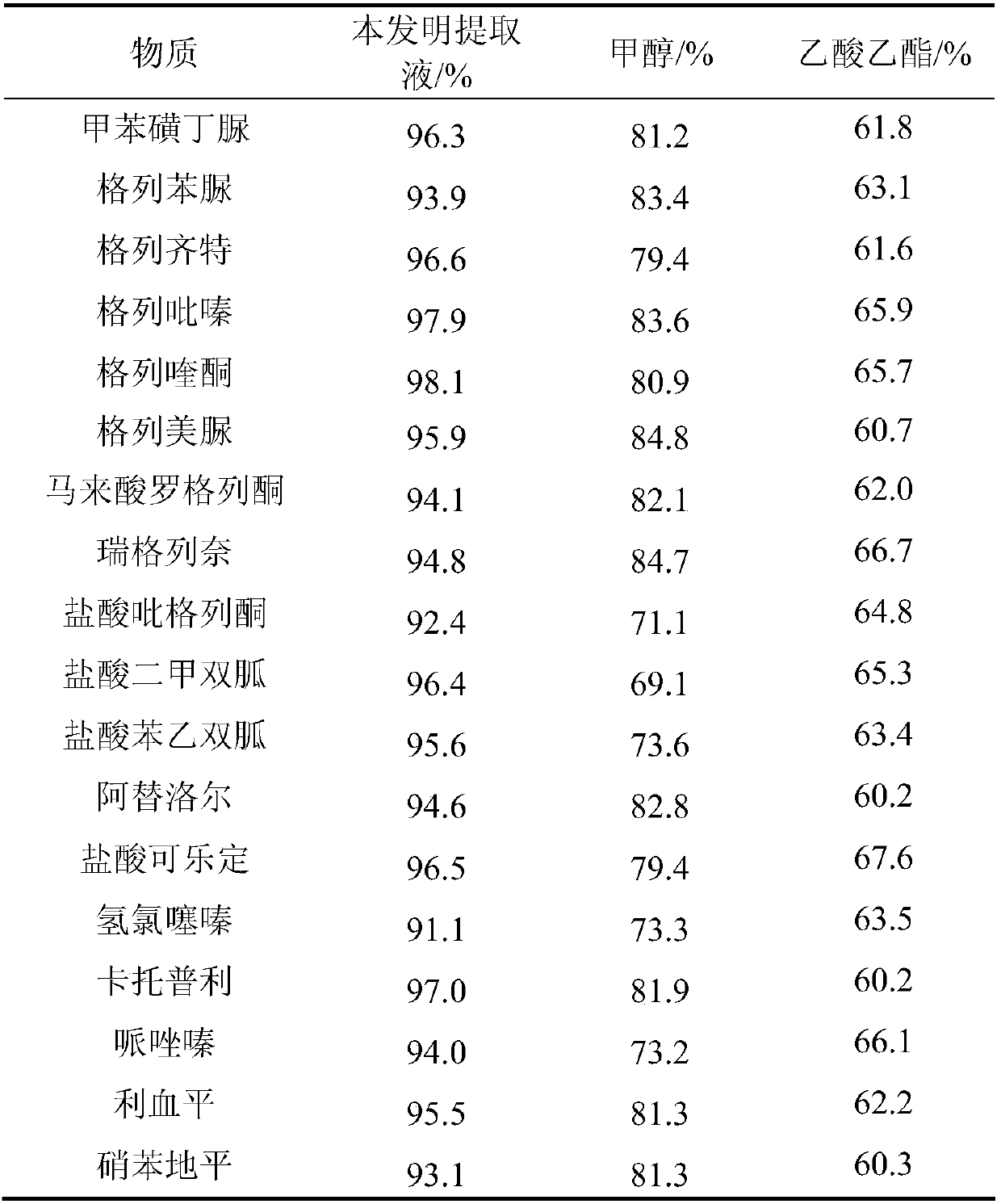
[0045] Prepare self-made extract in advance, mix 1000g of methanol, 100g of ethyl acetate and 1g of decyl alcohol polyoxyethylene ether, heat to reflux for 30min, cool and seal for storage until use.
[0046] Test a commercially available capsule health product (sample A); accurately weigh 5.00g of the sample into a 50mL plastic centrifuge tube with stopper, then add 30mL of self-made extract, and quickly mix it on a liquid mixer for 1min to make the sample Mix thoroughly. Ultrasonic extraction for 5min, centrifugation at 4000r / min for 10min, take 1mL of the supernatant and dry it with nitrogen at 40°C, reconstitute with 1mL of 5% acetonitrile aqueous solution; after passing through a 0.22μm microporous membrane, the filtrate is subjected to HPLC-MS / MS Determination, the results are shown in Table 3.
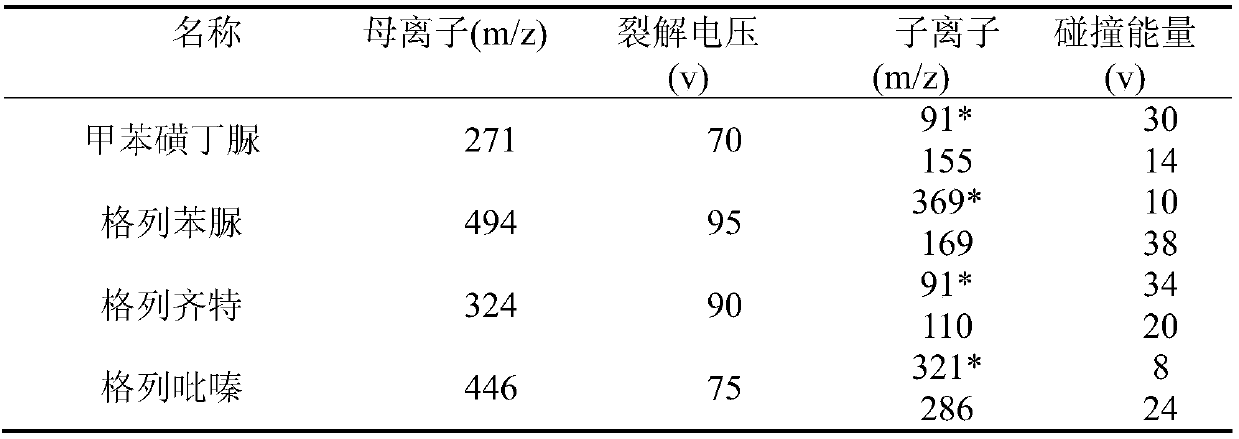
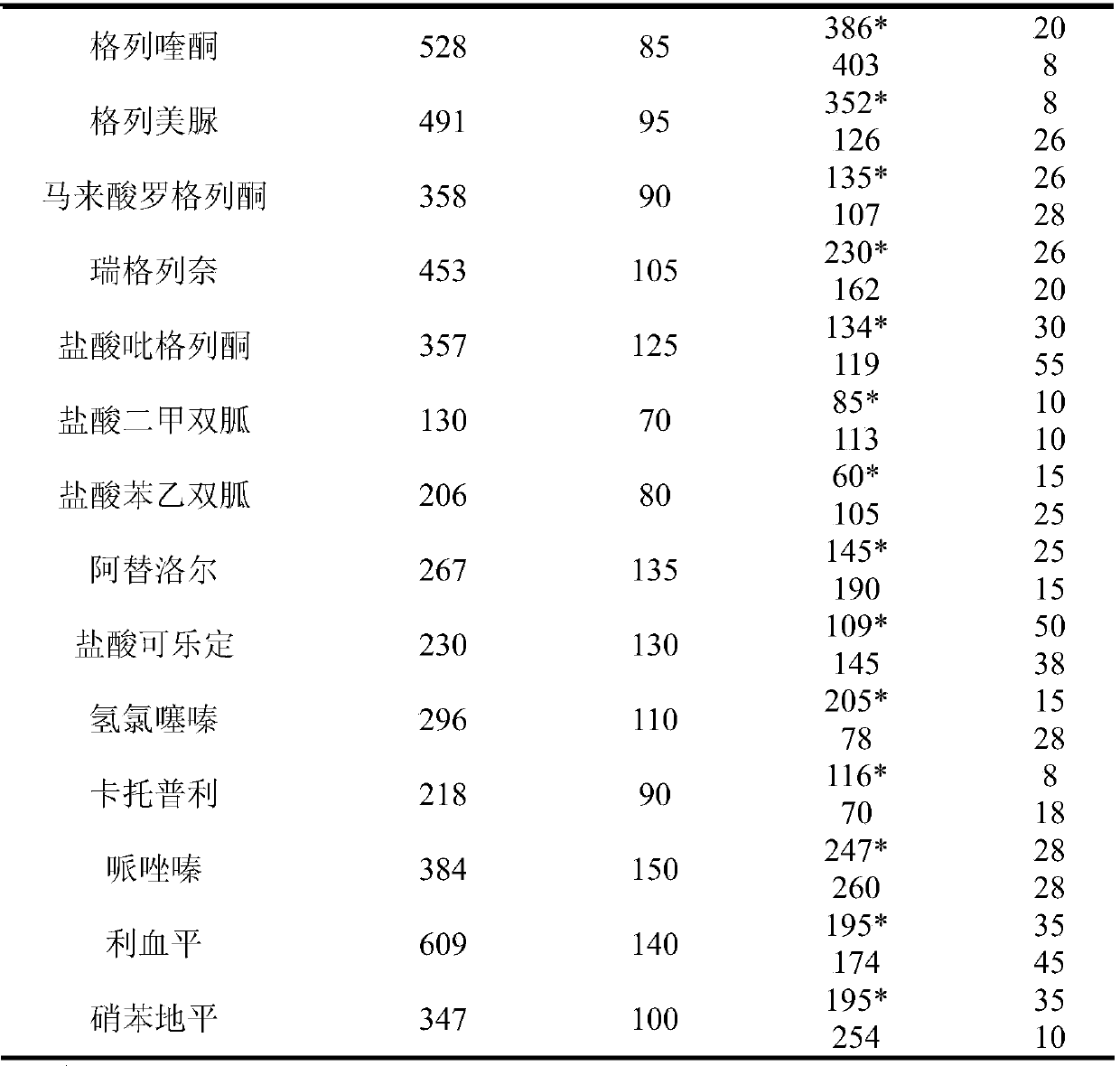
[0047] The detection conditions of HPLC-MS / MS are: the chromatographic conditions in the positive ion monitoring mode are: chromatographic column: Agilent ZORBAX SB-C18 column ...
Embodiment 2
[0051] Prepare self-made extract in advance, mix 1000g of methanol, 100g of ethyl acetate and 1g of decyl alcohol polyoxyethylene ether, heat to reflux for 30min, cool and seal for storage until use.
[0052] Test a commercially available granular health product (sample B); accurately weigh 5.00g of the sample into a 50mL plastic centrifuge tube with stopper, then add 30mL of self-made extract, and quickly mix it on a liquid mixer for 1min to make the test Mix completely. Ultrasonic extraction for 5min, centrifugation at 4000r / min for 10min, take 1mL of the supernatant and dry it with nitrogen at 40°C, reconstitute with 1mL of 5% acetonitrile aqueous solution; after passing through a 0.22μm microporous membrane, the filtrate is subjected to HPLC-MS / MS Determination, the results are shown in Table 3.
[0053] The detection conditions of HPLC-MS / MS are: in the positive ion monitoring mode, the chromatographic conditions are: chromatographic column: Agilent ZORBAX SB-C18 column ...
Embodiment 3
[0057] Prepare self-made extract in advance, mix 1000g of methanol, 100g of ethyl acetate and 1g of decyl alcohol polyoxyethylene ether, heat to reflux for 30min, cool and seal for storage until use.
[0058] Test a commercially available tablet-type health product (sample C); accurately weigh 5.00g of the sample into a 50mL plastic centrifuge tube with stopper, then add 30mL of self-made extract, and quickly mix it on a liquid mixer for 1min to make The sample was mixed thoroughly. Ultrasonic extraction for 5 minutes, centrifugation at 4000r / min for 10 minutes, 1 mL of the supernatant was blown dry with nitrogen at 40°C, and redissolved with 1 mL of 5% acetonitrile aqueous solution. After passing through a 0.22 μm microporous membrane, the filtrate was determined by HPLC-MS / MS, and the results are shown in Table 3.
[0059] The detection conditions of HPLC-MS / MS are: in the positive ion monitoring mode, the chromatographic conditions are: chromatographic column: Agilent ZORB...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com