Aminosilicone phthalocyanine-graphene oxide composite nanomaterial, its preparation method and application
A graphene composite and nanomaterial technology, applied in the directions of organic chemistry methods, chemical instruments and methods, wave energy or particle radiation treatment materials, etc. The effect of efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1, the preparation of amino silicon phthalocyanine (n=1)
[0052] Raw materials and reagents: Dichlorosilicon phthalocyanine, 2-(2-aminoethoxy)ethanol, potassium carbonate in a molar ratio of 1:6:6, the amount of pyridine is 9 ml / gram of dichlorosilicon phthalocyanine, toluene The dosage is 120 ml / gram of dichlorosilicon phthalocyanine.
[0053] In proportion, take dichlorosilicon phthalocyanine, 2-(2-aminoethoxy)ethanol, potassium carbonate (K 2 CO 3 ) and pyridine were dissolved in toluene, heated to 130°C, and refluxed for 18h under the protection of nitrogen. After the reaction solution was evaporated to remove the solvent in vacuo, the resulting solid was completely dissolved in chloroform, filtered, and washed three times with ultrapure water. After the filtrate was evaporated to dryness under reduced pressure, it was recrystallized four times with chloroform / n-hexane to obtain aminosilicone phthalocyanine (Formula I, n=1).
[0054] In the same way,...
Embodiment 2
[0055] Embodiment 2: Taking amino silicon phthalocyanine n=1 as an example, the reaction scheme of this embodiment is as follows:
[0056]
[0057]
[0058] The preparation method of amino silicon phthalocyanine-graphene oxide composite nanomaterial (PG), the steps are as follows:
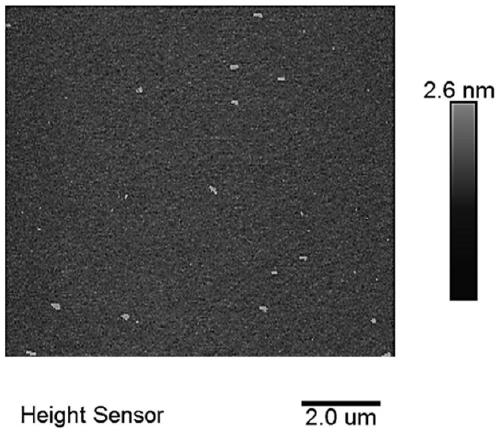
[0059] Add 9.6g of graphene oxide (GO) into 20L of distilled N,N-dimethylformamide, and sonicate for 3h. Add 72.6g (0.4M) 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, 43.7g (0.4M), N-hydroxysuccinyl Imine, 523mL (3M) N,N-diisopropylethylamine and 73.3g (0.1M) amino silicon phthalocyanine (n=1), stirred at room temperature, reacted under nitrogen atmosphere for 5 days. Dialyze the product, first use N,N-dimethylformamide as the dialysate, dialyze several times until the dialysate becomes colorless, and then use ultrapure water as the dialysate. Finally, the solid product was dispersed in ultrapure water and freeze-dried to obtain a total of 37.6 g of the product PG, including...
Embodiment 3
[0071] Example 3, preparation of amino silicon phthalocyanine-graphene oxide composite nanomaterial (PG, n=2)
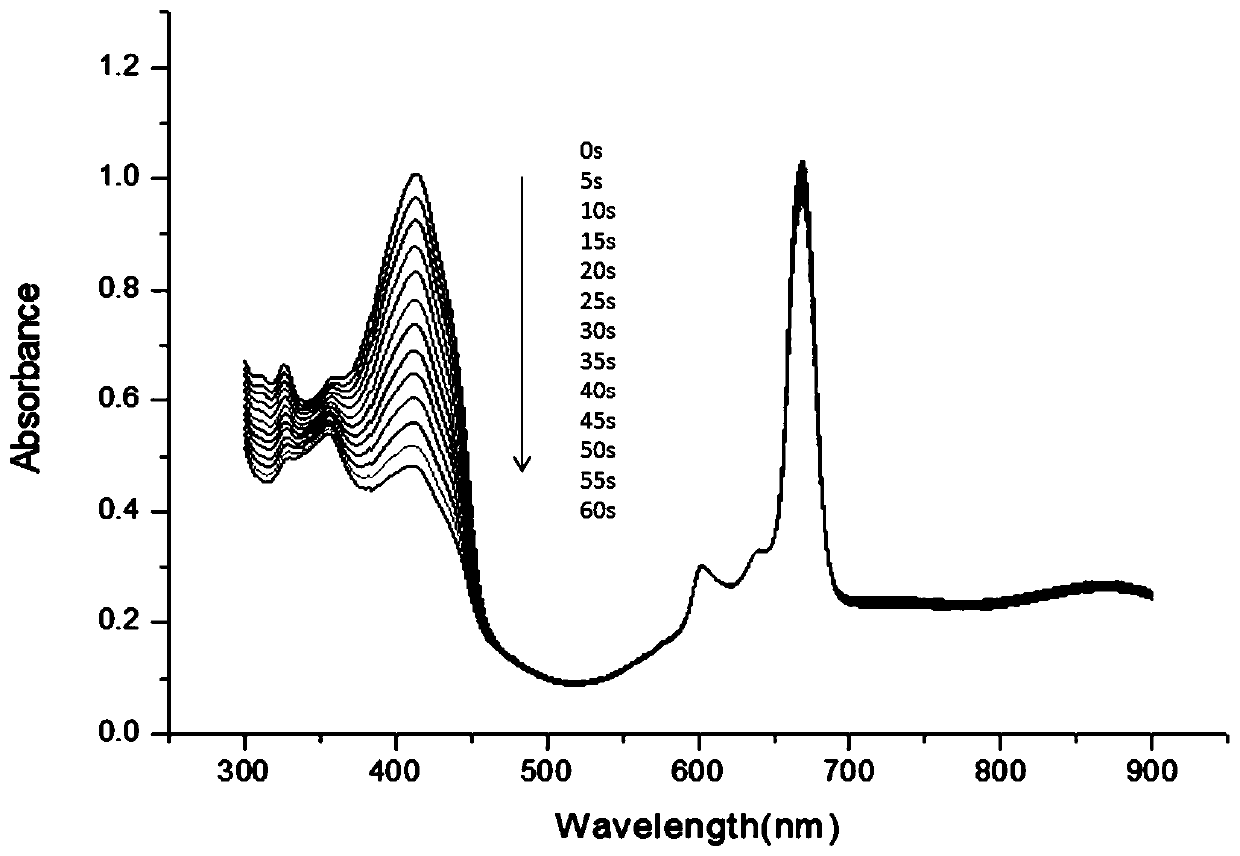
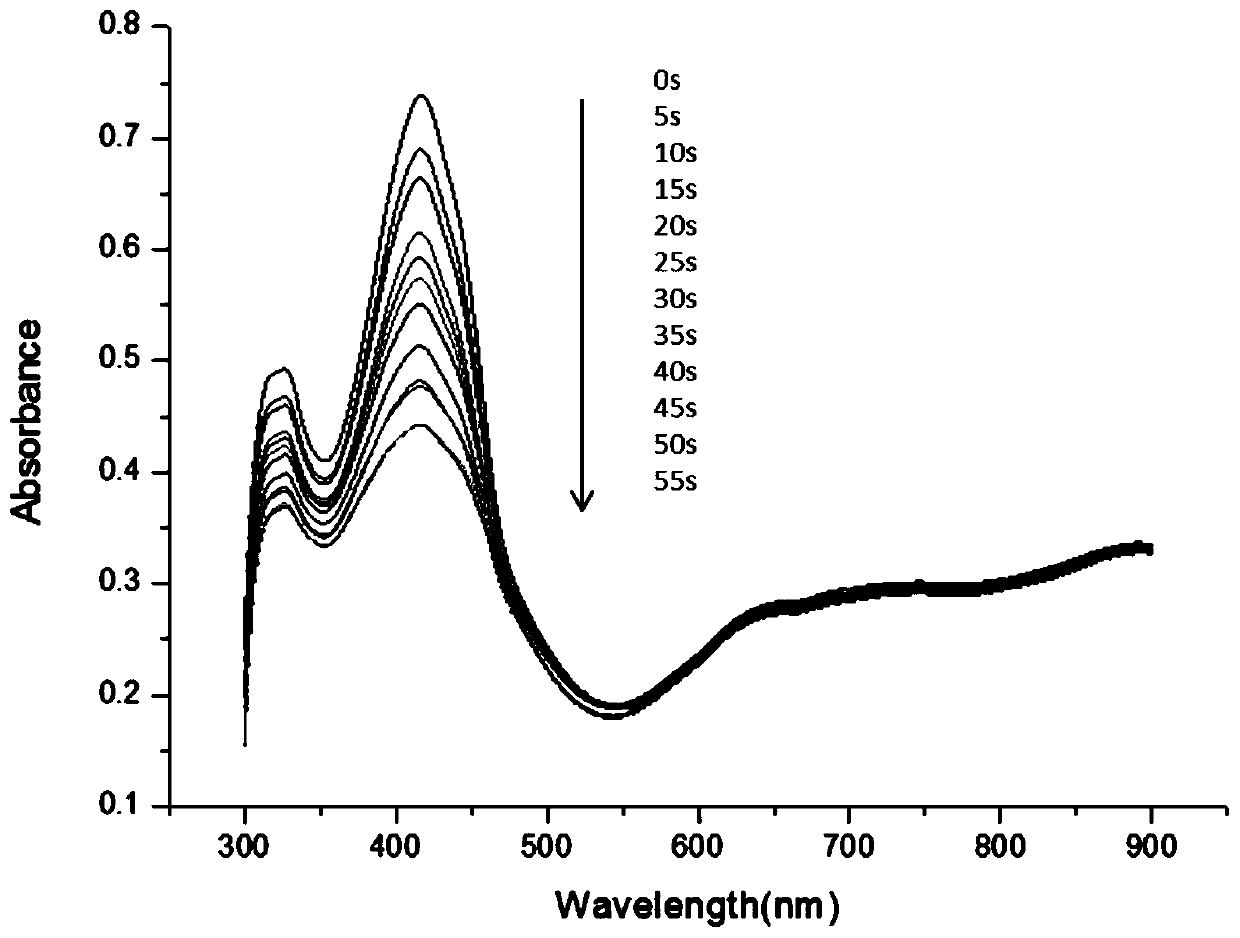
[0072] As described in Example 2, the difference is that the amino silicon phthalocyanine (n=1) is replaced by an equimolar amount of n=2 amino silicon phthalocyanine, the resulting product amino silicon phthalocyanine-oxidation Graphene composite nanomaterials tested by singlet oxygen in N,N-dimethylformamide and H 2 The yields of singlet oxygen in O are higher. And it has a very good thermotherapy effect, and it rises to about 42°C within 5 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com