An improved hereditary hemorrhagic telangiectasia related gene mutation detection kit
A technology of telangiectasia and detection kit, which is applied in the biological field, can solve the problems of non-containment and cumbersome methods, and achieve the effect of simple operation, simplified experimental steps, fast and effective detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] [Example 1] Preparation of blood sample DNA of the subject to be tested
[0053] 1. Research object
[0054] A 2-generation pedigree with 2 clinically diagnosed hereditary hemorrhagic telangiectasia was collected. The clinical features of the proband were repeated epistaxis, telangiectasia visible on the tip of the tongue, clear family history, hepatic hemangioma consistent with heredity Three of the clinical features of hemorrhagic telangiectasia can be diagnosed clinically; the son of the proband has the same disease. After signing the informed consent form, 5-10ml blood samples were collected from each person.
[0055] 2. Genomic DNA extraction
[0056] Using phenol chloroform extraction method.
[0057] first day
[0058] 1) Anticoagulant blood was diluted 1-fold with PBS.
[0059] 2) Add 2 times the volume of lymphatic separation solution (18°C to 28°C) into the centrifuge tube, spread a layer of 1 times the volume of diluted blood on top, centrifuge at 1000×g...
Embodiment 2
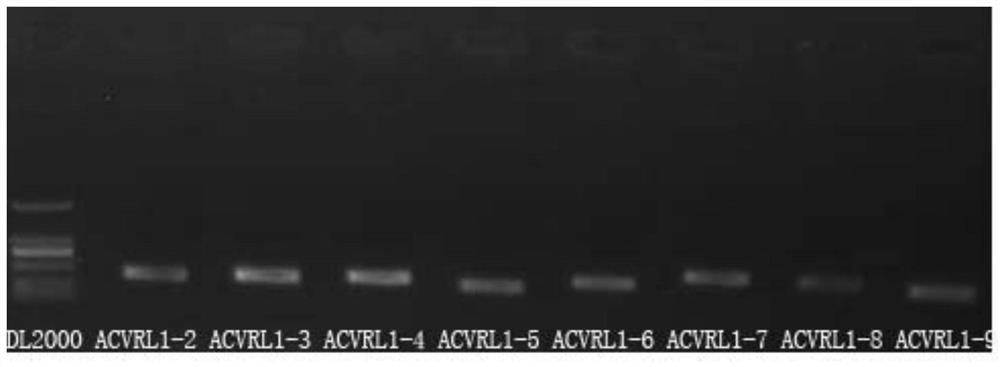
[0074] [Example 2] PCR amplification of ACVRL1, ENG, SMAD4 and BMP9 gene coding regions
[0075] 1. Primer sequence
[0076] The primers are the above-mentioned 37 pairs of specific primers, and the sequences are listed in Table 1.
[0077] 2. Establishment of PCR reaction system (Table 2)
[0078] Table 2 PCR reaction system
[0079]
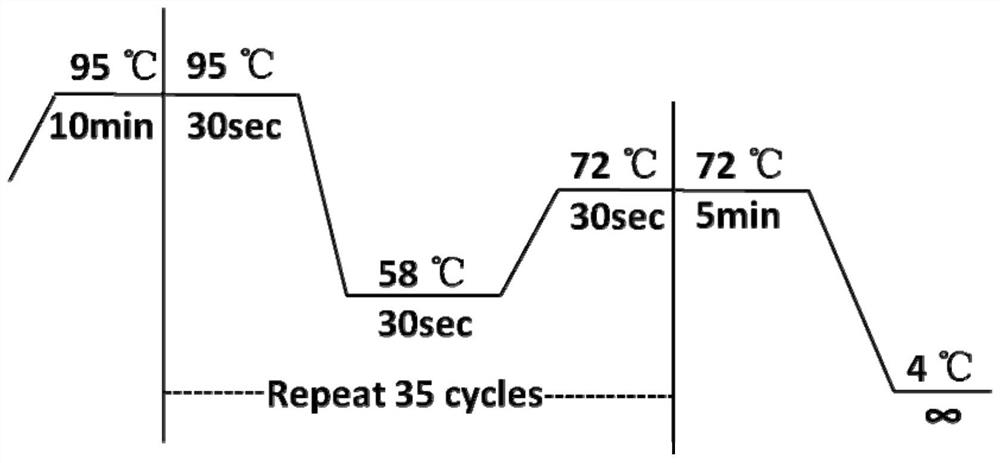
[0080] Reaction conditions: PCR reaction is carried out on the Mastercycler thermal cycler of eppendorf company, and the 37 pairs of specific primer reaction conditions that each sample carries out are consistent (comprising temperature and time), such as figure 1 shown.
Embodiment 3
[0081] [Example 3] Purification and quantification of PCR amplification products
[0082] 1. Purification of PCR products——96-well plate method
[0083] 1. Add 50 μl sterile water to the 96-well plate containing the PCR product and mix well.
[0084] 2. Transfer it to the Millipore purification plate, put it on the vacuum pump for about 3 minutes, and see that there is no water in the purification plate.
[0085] 3. Add 50 μl of deionized water to the purification plate again, and continue to filter until there is no water in the purification plate.
[0086] 4. Remove the purification plate from the vacuum pump, add 20 μl of deionized water to the plate, let it rest for 15 minutes, shake it for another 15 minutes, and then suck it into a new 96-well plate.
[0087] 5. Store in a -20°C refrigerator.
[0088] 2. Quantification by electrophoresis
[0089] 1. Sample preparation
[0090] Take a 96-well spotting plate, add 6 μl of sample buffer to each well, remove the PCR prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com