Method for preparing aromatic hydrocarbon by methyl alcohol
A technology for methanol preparation and aromatic hydrocarbons, which is applied in the purification/separation of hydrocarbons, chemical instruments and methods, and hydrocarbon production from oxygen-containing organic compounds, etc. Good technical effect, avoid cumulative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
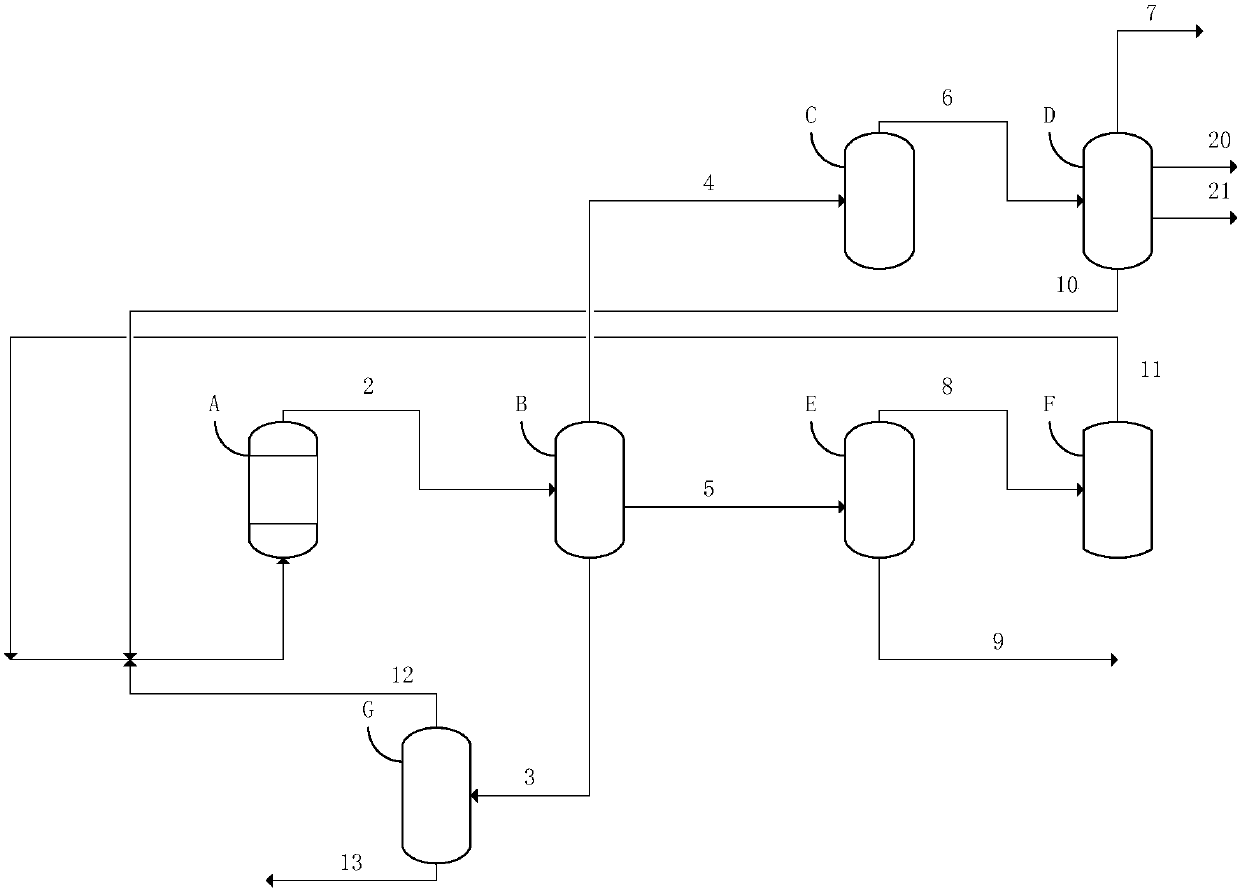
[0031] use figure 1 In the process flow shown, the methanol raw material is industrial methanol. The methanol aromatization reactor adopts a fluidized bed reactor, the aromatization catalyst used is a modified ZSM-5 molecular sieve, and the zinc oxide content of the auxiliary agent is 10% by weight. The reaction temperature is 400°C, the pressure is 0.1MPAG, and the weight space velocity is 0.2HR -1 . The reaction product was separated into gas phase, oil phase and water phase after cooling to 40°C. The gas phase is first washed with water in a water washing tower to remove oxygenated compounds. The liquid-to-gas mass ratio of the water washing tower is 3.2. The light hydrocarbon stream after water washing is separated from hydrogen and methane in the demethanizer, and the product hydrogen is separated by a pressure swing adsorption unit. Ethylene, ethane and light hydrocarbon recycle streams are separated in the deethanizer and ethylene rectification tower. Where ethylene...
Embodiment 2
[0034] use figure 1 In the process flow shown, the methanol raw material is industrial methanol. The methanol aromatization reactor adopts a fluidized bed reactor, the aromatization catalyst used is a modified ZSM-5 molecular sieve, and the zinc oxide content of the auxiliary agent is 10% by weight. The reaction temperature is 450°C, the pressure is 0.25MPAG, and the weight space velocity is 1.5HR -1 . The reaction product was separated into gas phase, oil phase and water phase after cooling to 40°C. The gas phase is firstly washed with water to remove oxygenated compounds in a water washing tower with a liquid-to-gas mass ratio of 3, and then the oxygenated compounds are further removed in a gas phase molecular sieve adsorber to obtain a light hydrocarbon stream, which is sent to the demethanizer, Separation into hydrogen, methane, ethylene, ethane and light hydrocarbon recycle streams in pressure swing adsorption unit, deethanizer and ethylene rectification tower. 50% of...
Embodiment 3
[0037] use figure 1 In the process flow shown, the methanol raw material is industrial methanol. The methanol aromatization reactor adopts a fluidized bed reactor, the aromatization catalyst used is a modified ZSM-5 molecular sieve, the zinc oxide content of the auxiliary agent is 10WT%, and the zinc nitrate content is 10WT%. The reaction temperature is 400°C, the pressure is 0.1MPAG, and the weight space velocity is 0.2HR -1 . The reaction product was separated into gas phase, oil phase and water phase after cooling to 40°C. The gas phase is firstly washed with water in a water washing tower to remove oxygenated compounds. The liquid-to-gas mass ratio of the water washing tower is 3.2. The light hydrocarbon stream after water washing is separated in the demethanizer, pressure swing adsorption unit, deethanizer and ethylene distillation tower Hydrogen, methane, ethylene, ethane and light hydrocarbon recycle streams. All of the ethylene is returned to the aromatization reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com