Non-aqueous chemical absorbent for separating and purifying carbon dioxide
A carbon dioxide, chemical absorption technology, applied in separation methods, dispersed particle separation, chemical instruments and methods, etc., can solve the problems of difficulty in maintaining the stability of the absorbent, complex system components, and high viscosity of the absorbent, and reduce sensible heat and The effect of latent heat of vaporization, high cycle absorption capacity and stable absorption performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
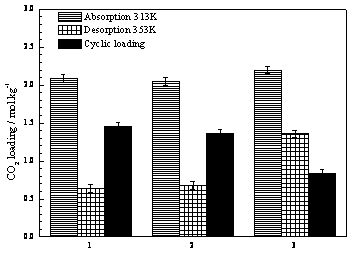
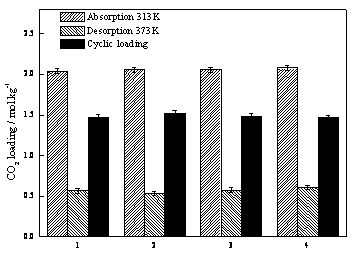
[0023] Add 100 grams of ethylene glycol butyl ether solution with a mass fraction of 20% ethanolamine to a split-type cold hydrazine with a height of 20 cm and an outer diameter of 3 cm, and place the device in a constant temperature water bath at 30 ° C; The flow rate is about 0.2 liters per minute, the gas is absorbed by bubbling in cold hydrazine, the exhaust gas passes through the grid snake condenser, and then enters the carbon dioxide analyzer after passing through the gas dryer to continuously measure the content at the outlet, and stop when the carbon dioxide content exceeds 23%. Experiment: Shake the absorption solution and accurately weigh 2.0 grams of the sample, transfer it to the Erlenmeyer flask, add 30 ml of water, add excess dilute sulfuric acid with a concentration of 0.3M, measure the volume of the released gas, and calculate the absorption load of carbon dioxide as The ratio of the number of moles of carbon dioxide in the solution to the weight of the absorbi...
Embodiment 2
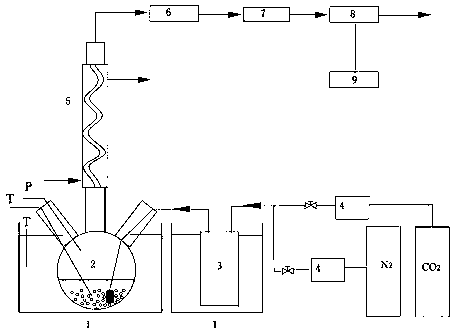
[0026] In the absorption-desorption experimental setup (see figure 1 ) into the mixed gas with a content of about 13% carbon dioxide (nitrogen is an inert gas), the flow rate is about 0.8 liters per minute, the device is placed in a constant temperature water bath at 40°C, and the gas enters the carbon dioxide analyzer continuously after passing through a disperser and a dryer. Measure the content of the discharge outlet, and after the outlet reading is stable, quickly add 150 grams of fresh absorbents prepared with different compositions, and the tail gas enters the carbon dioxide analyzer to continuously measure the content of the discharge outlet after passing through the grid snake condenser and gas dryer. Stop the experiment when the carbon dioxide content reaches 95% of the inlet concentration; shake the absorption solution and accurately weigh 2.0 grams of the sample, transfer it to a conical flask, add 30 ml of water, add excess dilute sulfuric acid with a concentration...
Embodiment 3
[0030] In the absorption-desorption experimental setup (see figure 1 ) into the mixed gas with a content of about 15% carbon dioxide (nitrogen is an inert gas), the flow rate is about 0.8 liters per minute, the device is placed in a constant temperature water bath at 40°C, and the gas enters the carbon dioxide analyzer continuously after passing through a disperser and a dryer. Measure the content of the discharge outlet, and after the outlet reading is stable, quickly add 150 grams of the prepared 30wt% MEA + 70wt% EGME non-water absorbent, and the exhaust gas passes through the grid snake condenser and gas dryer and then enters the carbon dioxide analyzer for continuous measurement. The content of the outlet, when the carbon dioxide content reaches 85% of the inlet concentration, the absorption experiment is stopped; after the absorption liquid is shaken, 2.0 grams of the sample is accurately weighed, and the absorption load of carbon dioxide is measured. Rapidly raise the t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com