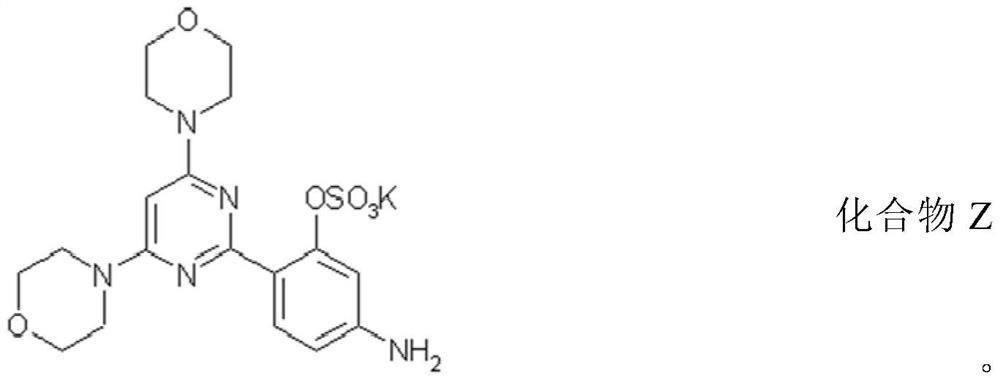
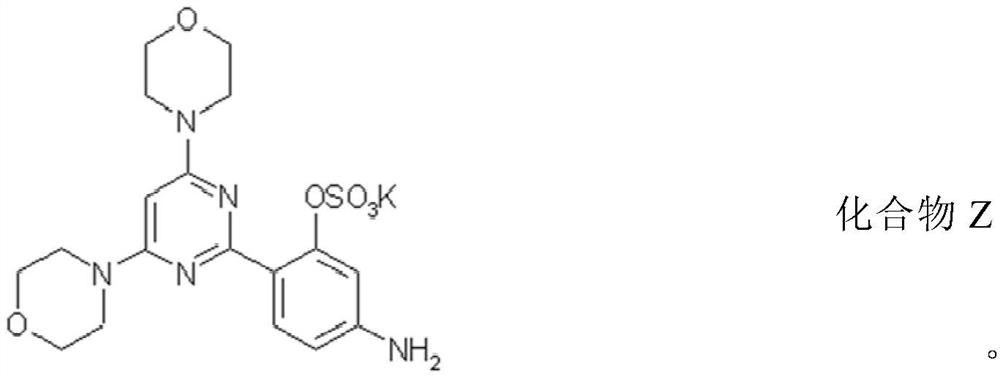
A kind of compound and its preparation method and use
A compound and composition technology, applied in the field of medicine, can solve problems such as increasing the risk of thrombosis in patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0075] The preparation method of the medical device described in the present invention includes but not limited to:
[0076] Dissolving compound X in a solvent and coating it on the surface of the medical device by direct spraying, dipping or a combination of the two methods;
[0077] Or use electrostatic spraying or anodic polarization to spray or dip-coat the surface of the bracket. Soak for 48 hours to stop adsorption, and freeze-dry in a vacuum dryer at -55°C.
[0078] The solvent of the drug is alkanes, alkenes, alcohols, aldehydes, amines, esters, ethers, ketones, aromatic hydrocarbons, hydrogenated hydrocarbons, terpene hydrocarbons, halogenated hydrocarbons, heterocyclic compounds, nitrogen-containing compounds and sulfur-containing compounds, etc., preferably acetic acid Ethyl ester, n-propyl acetate, acetone, tetrahydrofuran, chloroform, dichloromethane.
[0079]The medical device described in the present invention can be one or more of medical devices such as hear...
Embodiment 1
[0091] Preparation of compound W
[0092]
[0093] A: NH 3 / MeOH heating back for 1h
[0094] B: Phosgene / THF
[0095] C: POCl 3 , Morpholine heated to reflux for 5h
[0096] D: 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)aniline, Pd(Pcy 3 ) 2 Cl 2 , CsF, NMP-water (9:1) 100℃48h
[0097] E: carbamoyl chloride, TEA, DCM reflux for 36h
[0098] The product was confirmed by LCMS, new peak Rf=15.754, M+=398.
Embodiment 2
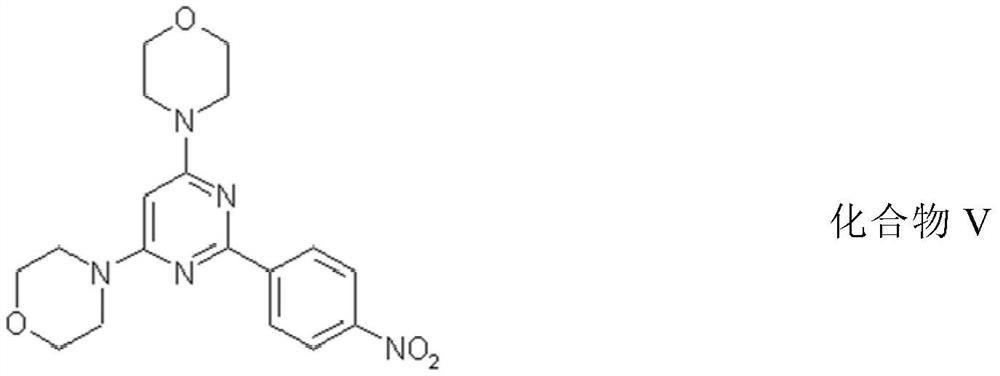
[0100] Preparation of compound V
[0101]
[0102] A: NH 3 / MeOH heating back for 1h
[0103] B: Phosgene / THF
[0104] C: POCl 3 , Morpholine heated to reflux for 5h
[0105] D: 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)aniline, Pd(Pcy 3 ) 2 Cl 2 , CsF, NMP-water (9:1) 100℃48h
[0106] E:CH 3 COOOH / CH 3 COOH
[0107] The product was confirmed by LCMS, new peak Rf=12.013, M+=371.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com