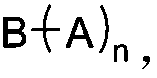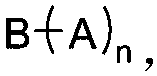Dynamic polymer thermoplastic elastomer and application thereof
A thermoplastic elastomer and polymer technology, used in applications, clothing, footwear, etc., can solve the problems of hard and brittle cross-linked polymers, inability to use elastomers, poor mechanical properties, etc. Recovery and reuse, excellent self-healing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
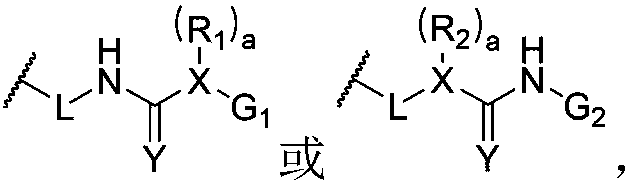
[0124] A method for preparing a dynamic polymer ionic liquid gel of the present invention comprises the following steps: blending the raw material for preparing the dynamic polymer with the ionic liquid so that the mass fraction of the raw material for preparing the dynamic polymer is 0.5 to 70%, and passing The appropriate means carry out polymerization, coupling or other types of chemical reactions, and after the reaction is completed, a dynamic polymer ionic liquid gel is produced. Another method for preparing a dynamic polymer ionic liquid gel of the present invention comprises the following steps: swelling the dynamic polymer in a solvent containing an ionic liquid so that the mass fraction of the dynamic polymer is 0.5-70%, and after fully swelling In addition to the solvent, a dynamic polymer ionic liquid gel is made. The above-mentioned ionic liquid is generally composed of organic cations and inorganic anions. As an example, the cations are selected from the group con...
Embodiment 1
[0168] Styrene-butadiene-styrene triblock copolymer (SBS), 2-(tert-butoxycarbonyl-amino)ethanethiol, photoinitiator benzyl dimethyl ketal (BDK) in tetrahydrofuran Reaction, keeping the molar ratio of alkenyl and 2-(tert-butoxycarbonyl-amino)ethanethiol and BDK in polybutadiene is about 50:50:1, and obtains polybutadiene segment containing carbamate base modified SBS.
[0169] 100 mass parts of gained modified SBS, 25 mass parts of naphthenic oil, 17 mass parts of light calcium carbonate, 25 mass parts of polystyrene, 8 mass parts of polyvinyl acetate, 2640.8 mass parts of antioxidant, 2 mass parts of zinc oxide part, 1.5 parts by mass of zinc stearate, and 2 parts by mass of AC foaming agent were uniformly mixed, extruded with a screw extruder, then kneaded on an open mill to go out into sheets, and foamed at 170°C and 20MPa for 8 minutes to form.
[0170] Performance and application: tensile strength 10.2MPa, elongation at break 710%; density: 87kg / m 3 ; This foam material ...
Embodiment 2
[0172] Dissolve 2-chlorocyclohexanone in dichloromethane, add m-chloroperoxybenzoic acid (mCPBA) 0.12mol, keep the molar ratio of 2-chlorocyclohexanone and mCPBA at 10:12, and react to obtain α-chloro-ε - caprolactone
[0173] Under anhydrous conditions, dissolve 50 molar equivalents of α-chloro-ε-caprolactone and 100 molar equivalents of ε-caprolactone in toluene, and in 1 molar equivalent of initiator 2,2-dibutyl-2-tin-1 , 3-Dioxetane triggers the reaction at 20 ° C to obtain a polyester-based random copolymer 2a containing chlorine atoms at both ends of the hydroxyl-terminated side group.
[0174] The copolymer 2a whose side group contains chlorine atoms is dissolved in dimethylformamide, and sodium azide with 2 molar equivalents of chlorine atoms is added to react to obtain a copolymer whose side groups contain azide groups.
[0175] Dissolve the copolymer containing azide group in the side group, 2-propargyl-N-butyl carbamate with equimolar equivalent of azido group in t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com