Fascaplysin derivative as well as preparation method and application
A compound and drying technology, applied in the fields of drug combination, organic chemistry, antitumor drugs, etc., can solve the problems of fascaplysin's high cytotoxicity, limited clinical application, large toxic and side effects, etc., and achieves low price, good water solubility and short preparation route. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] 1. Preparation of compounds
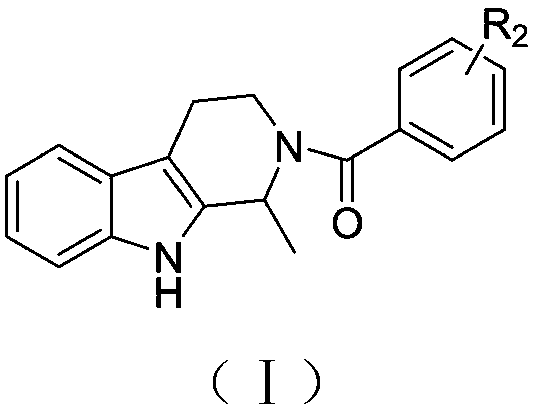
[0018] (1) Preparation of the compound of formula (I)
[0019]
[0020] 1. Weigh 2mmol of tryptamine and dissolve it in 20ml of water, stir at room temperature, add 3mmol of concentrated sulfuric acid drop by drop, the solution is clarified, take 20mmol of 40% acetaldehyde solution, dissolve it in 5ml of water and slowly add it into the reaction solution, stir at room temperature for 30min, 100 Reflux at ℃ for 7 hours, cool the reaction solution to room temperature, adjust the pH to 10 with aqueous sodium hydroxide solution, extract with dichloromethane, combine the organic phases, rinse with brine, dry with anhydrous sodium sulfate, filter and distill under reduced pressure, and purify through a chromatographic column (Dichloromethane:petroleum ether:methanol=5:2:1; 1% triethylamine) The target intermediate compound 1-methyl-1,2,3,4-tetrahydro-β-carboline was obtained.
[0021] 2. Weigh the corresponding acid (1.8mmol) and dissolve it ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com