Method for tubular reaction preparation of substituted benzylically brominated methyl biphenyl and reaction device of method
A technology of methyl biphenyl and reaction device, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid nitrile, etc., can solve problems such as difficult separation and extraction, long reaction time, low conversion rate, etc., and achieve reaction control Simple, convenient after-treatment, and less by-product formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
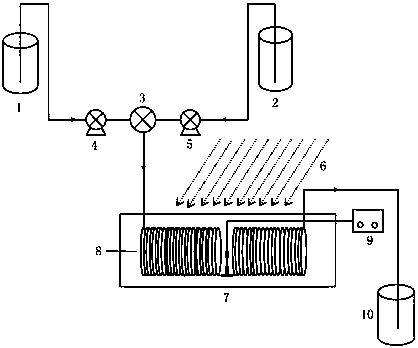
[0035] The structure of the special reaction device used in this embodiment is as follows: figure 1 As shown, the reactor 8 adopts a pipeline with a length of 30m and a diameter of 2mm, and the material of the pipeline is a polytetrafluoroethylene tube; the light source 6 is a UV lamp with a wavelength of 250nm.
[0036] First turn on the light source 6, turn on the internal circulation constant temperature water bath 7 to preheat the pipeline, add 100mL of water to the receiving tank 10, and set the reaction temperature to 77°C; Tetrazolium]-4-methylbiphenyl and 12.8 g (0.08 mol) of bromine were dissolved in 480 mL of ethyl acetate and stored in the storage tank a1; 17.5 g of 30% hydrogen peroxide (0.15 mol) was added to the storage solution In the tank b2, turn on the infusion pump a4 and the infusion pump b5, adjust the flow rate, and continuously input 2'-[(N-trityl)tetrazolium]-4-methylbiphenyl, bromine and hydrogen peroxide into the tank b2 respectively Mix in the high-...
Embodiment 2
[0038] The structure of the reaction device is as figure 1 , the reactor 8 adopts a pipeline with a length of 50m and a diameter of 2mm, and the material of the pipeline is a polytetrafluoroethylene tube; the light source 6 is a UV lamp with a wavelength of 250nm.
[0039] First turn on the light source 6, turn on the internal circulation constant temperature water bath 7 to preheat the pipeline, add 100mL of water to the receiving tank 10, and the reaction temperature is 78°C; Azole]-4-methylbiphenyl and 32.0g (0.2mol) bromine were dissolved in 240mL ethyl acetate and stored in the liquid storage tank a1 and liquid storage tank b2 respectively, and the infusion pump a4 and the infusion pump b5 were turned on , adjust the flow rate, continuously input 2′-[(N-trityl)tetrazolium]-4-methylbiphenyl and bromine into the high-efficiency mixer 3 for mixing, and then enter the internal circulation constant temperature water bath React in the pipeline of 7 inner light, reaction time 1...
Embodiment 3
[0041] The structure of the reaction device is as figure 1 , the reactor 8 adopts a pipeline with a length of 100 m and a diameter of 2 mm, and the material of the pipeline is a polytetrafluoroethylene tube; the light source 6 is a 250nm UV lamp.
[0042] First turn on the light source 6, turn on the internal circulation constant temperature water bath 7 to preheat the pipeline, add 100mL water to the receiving tank 10, and the reaction temperature is 78°C; Azole]-4-methylbiphenyl and 32.0g (0.2mol) bromine were respectively dissolved in 240mL ethyl acetate and stored in liquid storage tank a1 and liquid storage tank b2 respectively, and the infusion pump a4 and infusion pump b5 were turned on, Adjust the flow rate, respectively input 2'-[(N-trityl)tetrazolium]-4-methylbiphenyl and bromine continuously into the high-efficiency mixer 3 for mixing, and then enter the inner circulation constant temperature water bath 7 Carry out the reaction in the pipeline of internal light, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com