Ribociclib synthesizing method
A ribociclib and compound technology, applied in the field of drug synthesis, can solve the problems of complex synthesis, high cost, low yield and the like, and achieve the effects of good reaction selectivity, fewer synthesis steps and lower production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
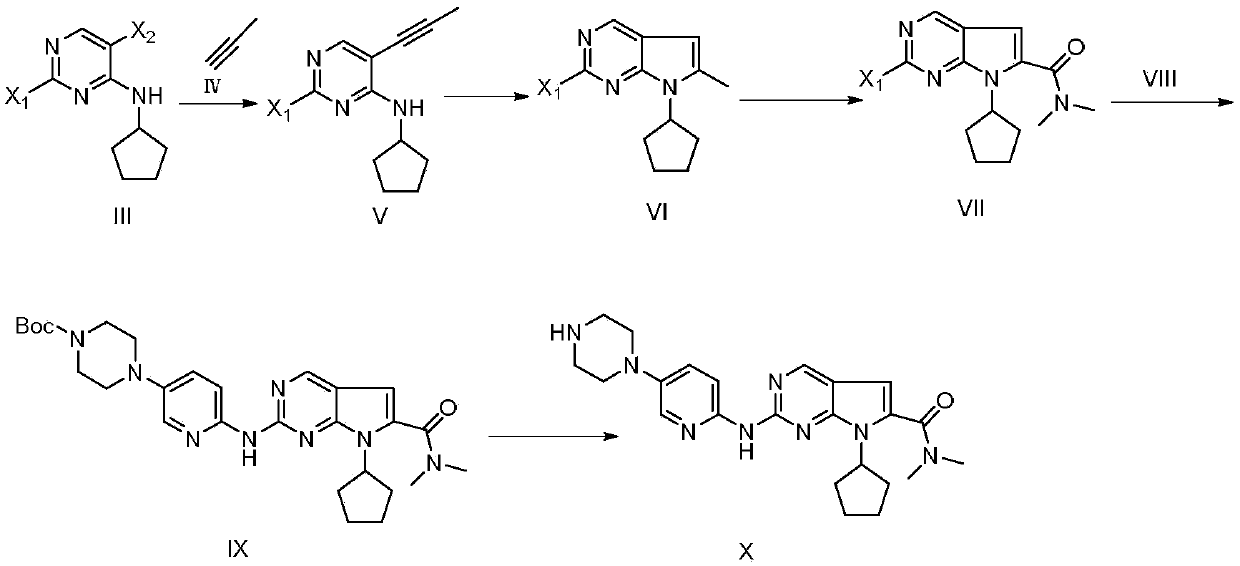
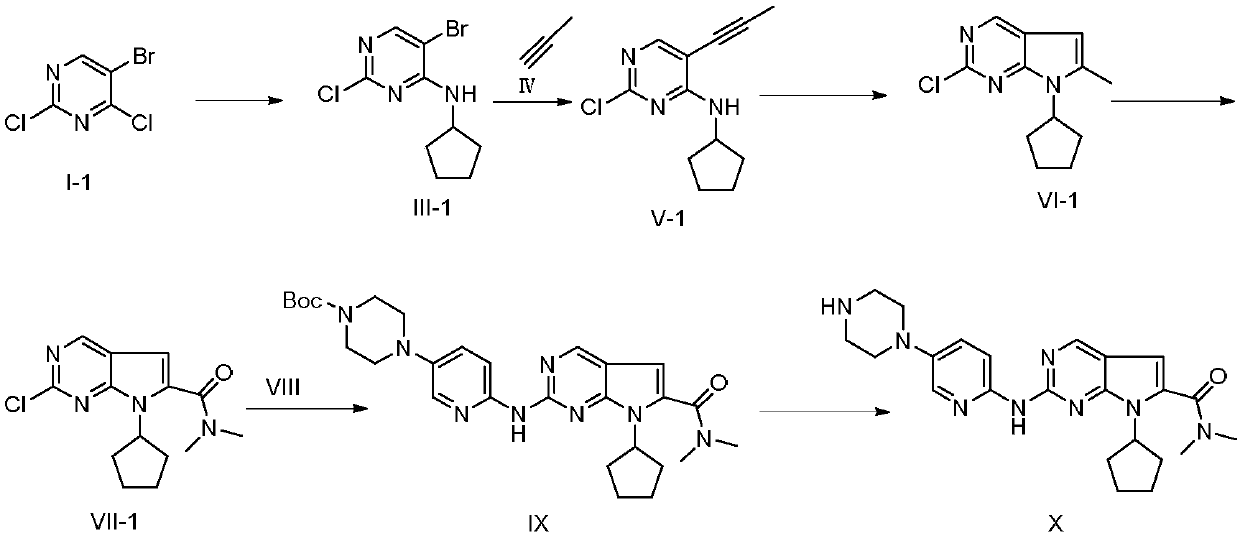
[0051] (1) Preparation of 2-chloro-4-cyclopentylamino-5-bromopyrimidine (III-1)
[0052] Add 5-bromo-2,4-dichloropyrimidine (I) (11.4g, 50mmol), triethylamine (2.24g, 20mmol) and dichloromethane (150mL) into the reaction flask, stir well and cool to 0~ At 5°C, cyclopentylamine (II) (4.257g, 50mmol) was slowly added dropwise, and after the dropwise addition, the temperature was raised to 45°C to react for 6 hours, and TLC detected that the reaction was complete. Add 150 mL of water to quench the reaction, wash the organic phase twice with saturated brine, extract the aqueous phase twice with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, and recover the solvent by distillation under reduced pressure. The compound 2-chloro-4-cyclopentylamino-5-bromopyrimidine (III-1) was separated by column chromatography in ethyl acetate mixed solvent 11.0g; the yield was 80%; the purity was 99.8% (HPLC area normalization method) ; Mass spectrum (ESI): m / z = 275....
Embodiment 2
[0064] (1) Preparation of 2-chloro-4-cyclopentylamino-5-bromopyrimidine (III-1)
[0065] Same as Example 1, except that the molar ratio of compound 5-bromo-2,4-dichloropyrimidine to cyclopentylamine is 1:1; the reaction temperature is 40°C. The base used is pyridine; the molar ratio of the compound 5-bromo-2,4-dichloropyrimidine to the base is 1:0.2; the reaction solvent is dimethylformamide.
[0066] (2) Preparation of 2-chloro-4-cyclopentylamino-5-(propynyl)pyrimidine (V-1)
[0067] Same as Example 1, except that the molar ratio of 2-chloro-4-cyclopentylamino-5-bromopyrimidine (III-1) to catalyst is 1:0.1. The ligand is selected from triphenylphosphine, and the molar ratio of 2-chloro-4-cyclopentylamino-5-bromopyrimidine (III-1) to the ligand is 1:0.1. The solvent used was dimethylformamide; the reaction temperature was 50°C.
[0068] Palladium chloride (1.063g, 6mmol) was used as a catalyst, and the final compound 2-chloro-4-cyclopentylamino-5-(propynyl)pyrimidine (V-1) ...
Embodiment 3
[0081] (1) Preparation of 2-chloro-4-cyclopentylamino-5-bromopyrimidine (III-1)
[0082] Same as Example 1, except that the molar ratio of compound 5-bromo-2,4-dichloropyrimidine to cyclopentylamine is 1:2; the reaction temperature is 100°C. The base used is N-methylmorpholine; the molar ratio of the compound 5-bromo-2,4-dichloropyrimidine to the base is 1:2; the reaction solvent is dimethyl sulfoxide.
[0083] (2) Preparation of 2-chloro-4-cyclopentylamino-5-(propynyl)pyrimidine (V-1)
[0084] Same as Example 1, except that the molar ratio of 2-chloro-4-cyclopentylamino-5-bromopyrimidine (III-1) to catalyst is 1:0.3. The ligand is selected from tri-tert-butylphosphine. The molar ratio of 2-chloro-4-cyclopentylamino-5-bromopyrimidine (III-1) to ligand is 1:0.5. The solvent used was dimethylsulfoxide; the reaction temperature was 110°C.
[0085] Cuprous iodide (1.1427g, 6mmol) was used as a catalyst, and the final obtained compound 2-chloro-4-cyclopentylamino-5-(propynyl)py...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com