Method for synthesizing 3,4-dimethoxythiophene
A technology of dimethoxythiophene and methoxythiophene, which is applied in the field of synthesizing 3,4-dimethoxythiophene, can solve the problems of environmental pollution, low product yield, high production cost, etc., and achieve reduction of environmental pollution, reaction The effect of low temperature and high utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
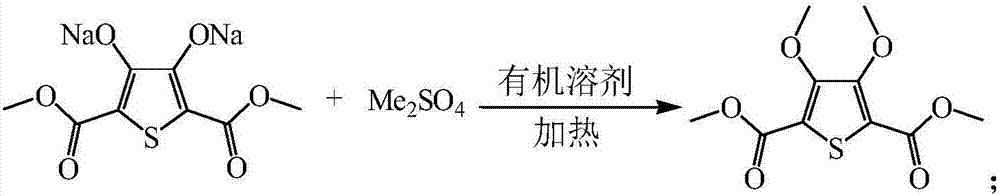
[0028] Add 293.2g of ethylene glycol dimethyl ether to 146.6g of 3,4-dihydroxythiophene-2,5-dicarboxylate dimethyl disodium salt, add 80.3g of dimethyl sulfate dropwise when heated to 70°C, and In the process of adding dimethyl sulfate dropwise, sodium hydroxide is added to make the pH value of the reaction system at 8-10. After adding dimethyl sulfate, the temperature is raised to 85°C, and the reaction is kept for 5 hours. After the reaction, ethylene glycol dimethyl ether is recovered. Add water to make a slurry, and filter to obtain 120.4g of dimethyl 3,4-methoxythiophene-2,5-dicarboxylate, detected by gas chromatography, with a yield of 87.2%;
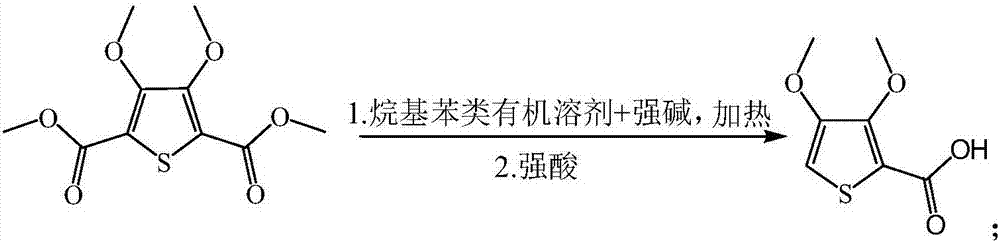
[0029] Add 280mL of dodecylbenzene to the 3,4-dimethoxythiophene-2,5-dicarboxylic acid dimethyl ester obtained in the above reaction, and then add a 50 wt% sodium hydroxide aqueous solution to make the reaction system When the pH is 12-14, heat to 200° C., stir and react for 3 hours. After the reaction is completed, acidify to a p...
Embodiment 2
[0035] Add 713.5g N,N-dimethylformamide to 146.3g 3,4-dihydroxythiophene-2,5-dicarboxylate dimethyl disodium salt, add 200.4g dimethyl sulfate dropwise when heated to 120°C In the process of adding dimethyl sulfate dropwise, sodium carbonate is added to make the pH value of the reaction system at 8.5. After adding dimethyl sulfate, the temperature is raised to 150°C, and the reaction is kept for 2 hours; after the reaction, N,N-dimethylformaldehyde is recovered Amide, beating with water, and filtering to obtain 122.4 g of dimethyl 3,4-methoxythiophene-2,5-dicarboxylate. The yield was 88.8% as detected by gas chromatography.
[0036]Add 711.6mL of diamylbenzene to the 3,4-dimethoxythiophene-2,5-dicarboxylic acid dimethyl ester obtained in the above reaction, and then add an aqueous potassium hydroxide solution with a mass fraction of 50wt% to make the reaction system pH at 12-14, heated to 180°C, stirred and reacted for 5 hours, after the reaction, acidified with 50 wt% nitric ...
Embodiment 3
[0042] Add 550g xylene to 150.3g 3,4-dihydroxythiophene-2,5-dicarboxylate dimethyl disodium salt, add 117.3g dimethyl sulfate dropwise when heated to 50°C, add dimethyl sulfate dropwise Add sodium carbonate during the esterification process to make the pH value of the reaction system at 8-10. After adding dimethyl sulfate, raise the temperature to 120°C and keep it warm for 2 hours. After the reaction, recover xylene, add water to make a slurry, and filter to obtain the product 3,4- 124.0 g of dimethyl methoxythiophene-2,5-dicarboxylate, detected by gas chromatography, the yield was 87.6%.
[0043] Add 433 mL of n-pentylbenzene to the 3,4-dimethoxythiophene-2,5-dicarboxylic acid dimethyl ester obtained in the above reaction, and then add an aqueous sodium hydroxide solution with a mass fraction of 50 wt % to make the pH of the reaction system at 12-14, heated to 150°C, stirred and reacted for 8 hours, after the reaction was completed, acidified with a sulfuric acid aqueous sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com