Oligomeric anion sulfonate surfactants, and preparation method and application thereof
An anionic sulfonate and surfactant technology, applied in the preparation of sulfonate, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of anhydrous protection, reduced yield, and high reaction risk.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Dipolyanion sulfonate DMEDA-(C 12 SO 3 Na) 2 Preparation of:
[0073]
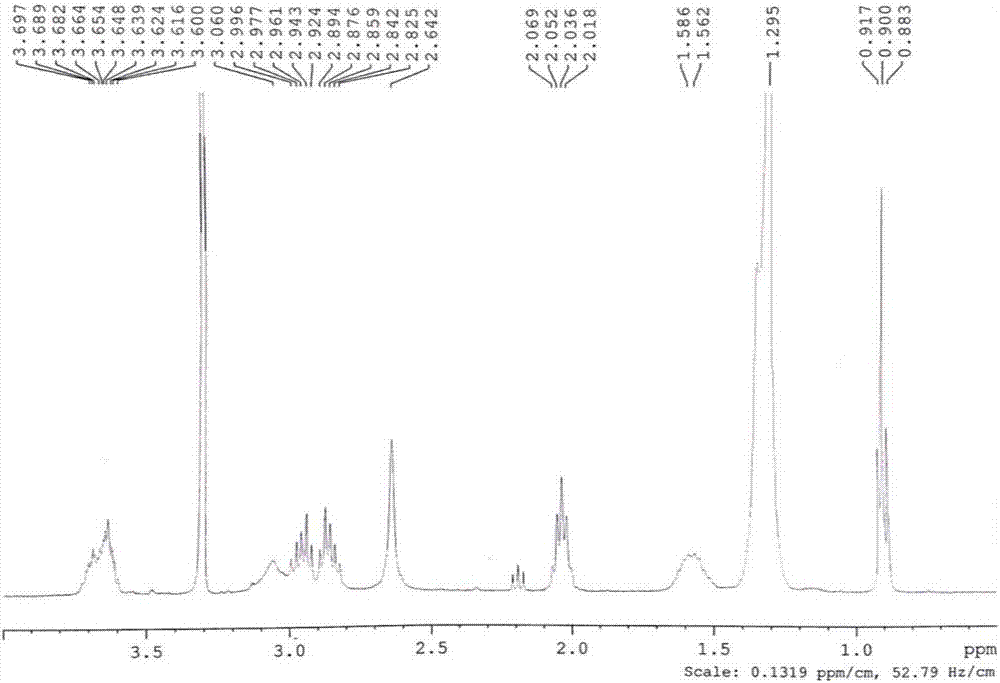
[0074] (1) Synthesis steps of the intermediate product dimer alcohol: add 0.9g (10.0mmol) of N,N'-dimethylethylenediamine to 6.4g (30.0mmol) of 1,2-epoxytetradecane Ethanol (50ml) solution was reacted at 90°C for 24h. After the reaction, use a rotary evaporator to remove the absolute ethanol solvent, dissolve the crude product in chloroform, and then pass through a silica gel chromatography column with chloroform / methanol for purification and vacuum drying to obtain the intermediate dimer alcohol.
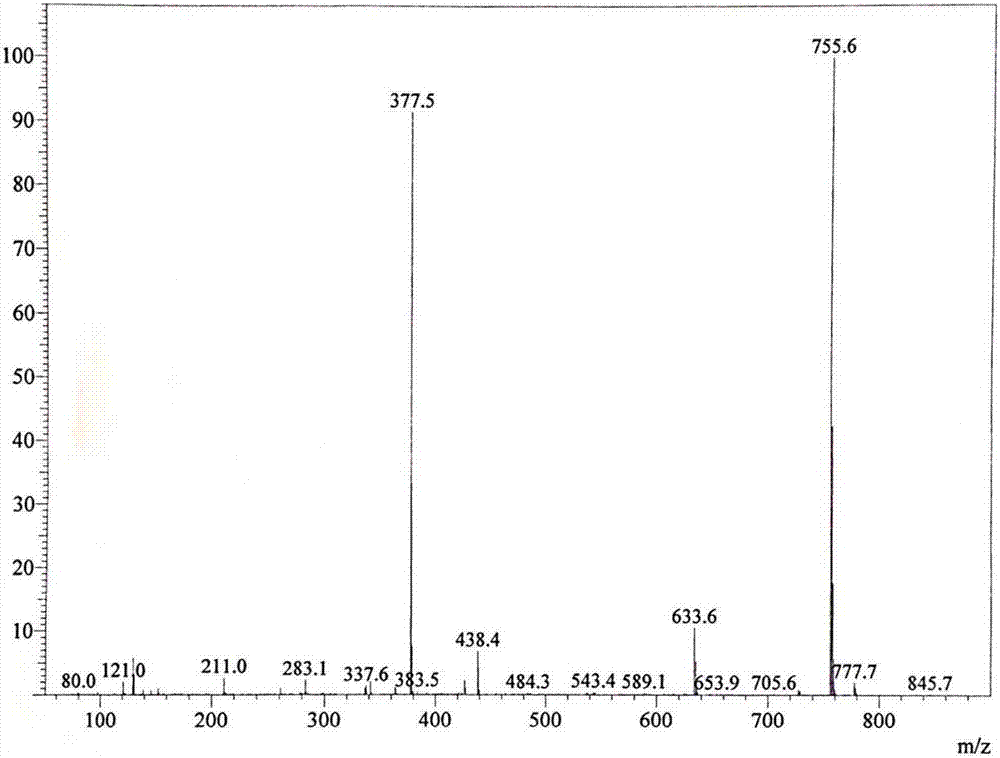
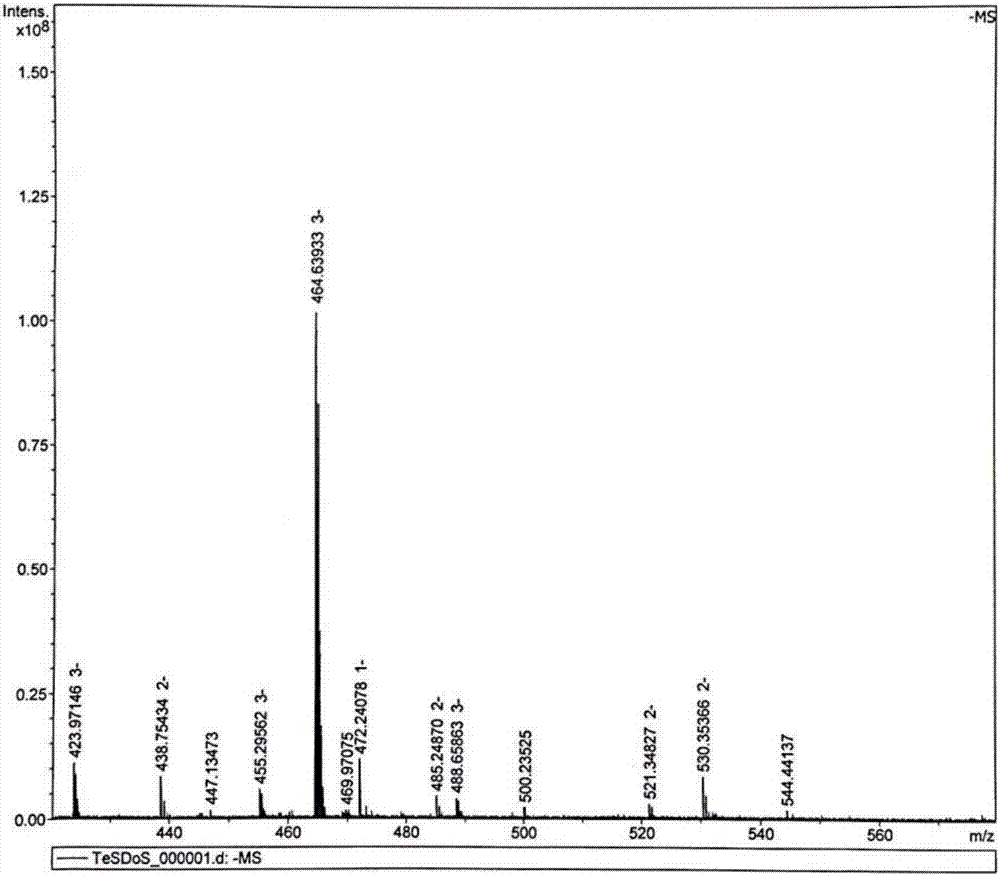
[0075] The intermediate dimer alcohol was characterized by MALDI-TOF: 513.5 (M+H).
[0076] (2) Dipolyanionic sulfonate surfactant DMEDA-(C 12 SO 3 Na) 2 The synthesis steps of the method: take 0.3g (60%, 7.5mmol) of sodium hydride, 1.0g (8.2mmol) of 1,3-propane sultone, and 0.5g (1.0mmol) of the intermediate dimer alcohol, dissolve them in THF, and reflux 48h, then add methanol to end the rea...
Embodiment 2
[0079] Tetrapolyanionic sulfonate surfactant EDA-(C 12 SO 3 Na) 4 Preparation of:
[0080]
[0081] (1) Synthetic steps of the intermediate product tetramer alcohol: ethylenediamine 0.6g (10.0mmol) is added to 1,2-epoxytetradecane 12.7g (60.0mmol) in dehydrated ethanol (50ml) solution at 90 Reaction at ℃ for 24h. After the reaction, the absolute ethanol solvent was removed with a rotary evaporator, and the crude product was dissolved in chloroform, then eluted with chloroform / methanol through a silica gel chromatography column, refined and vacuum-dried to obtain white powdery intermediate IV Polyol, 99% yield.
[0082] MALDI-TOF characterizes the intermediate tetramer: 910.1 (M+H).
[0083] (2) Tetrapolyanionic sulfonate surfactant EDA-(C 12 SO 3 Na) 4 The synthesis steps of the method: take 0.6g (60%, 15.0mmol) of sodium hydride, 2.0g (16.4mmol) of 1,3-propane sultone, and 0.9g (1.0mmol) of the intermediate tetramer alcohol, dissolve them in THF, and reflux 48h, t...
Embodiment 3
[0086] Hexameric anionic sulfonate surfactant TAEA-(C 12 SO 3 Na) 6 Preparation of:
[0087]
[0088] (1) Synthetic steps of the intermediate product hexamerol: the dehydrated alcohol ( 50ml) solution at 90°C for 24h. After the reaction, use a rotary evaporator to remove the absolute ethanol solvent, dissolve the crude product in chloroform, and then pass through a silica gel chromatography column with chloroform / methanol for purification and vacuum drying to obtain the intermediate hexamerol.
[0089] The intermediate hexamer alcohol was characterized by MALDI-TOF: 1420.6 (M+H).
[0090] (2) Hexameric anionic sulfonate surfactant TAEA-(C 12 SO 3 Na) 6 The synthesis steps of the method: take 1.0g (60%, 25mmol) of sodium hydride, 2.9g (23.7mmol) of 1,3-propane sultone, and 1.4g (1.0mmol) of the intermediate hexamerol, dissolve them in THF, and reflux for 48h , and then methanol was added to terminate the reaction. The solvent was removed by rotary evaporation, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com