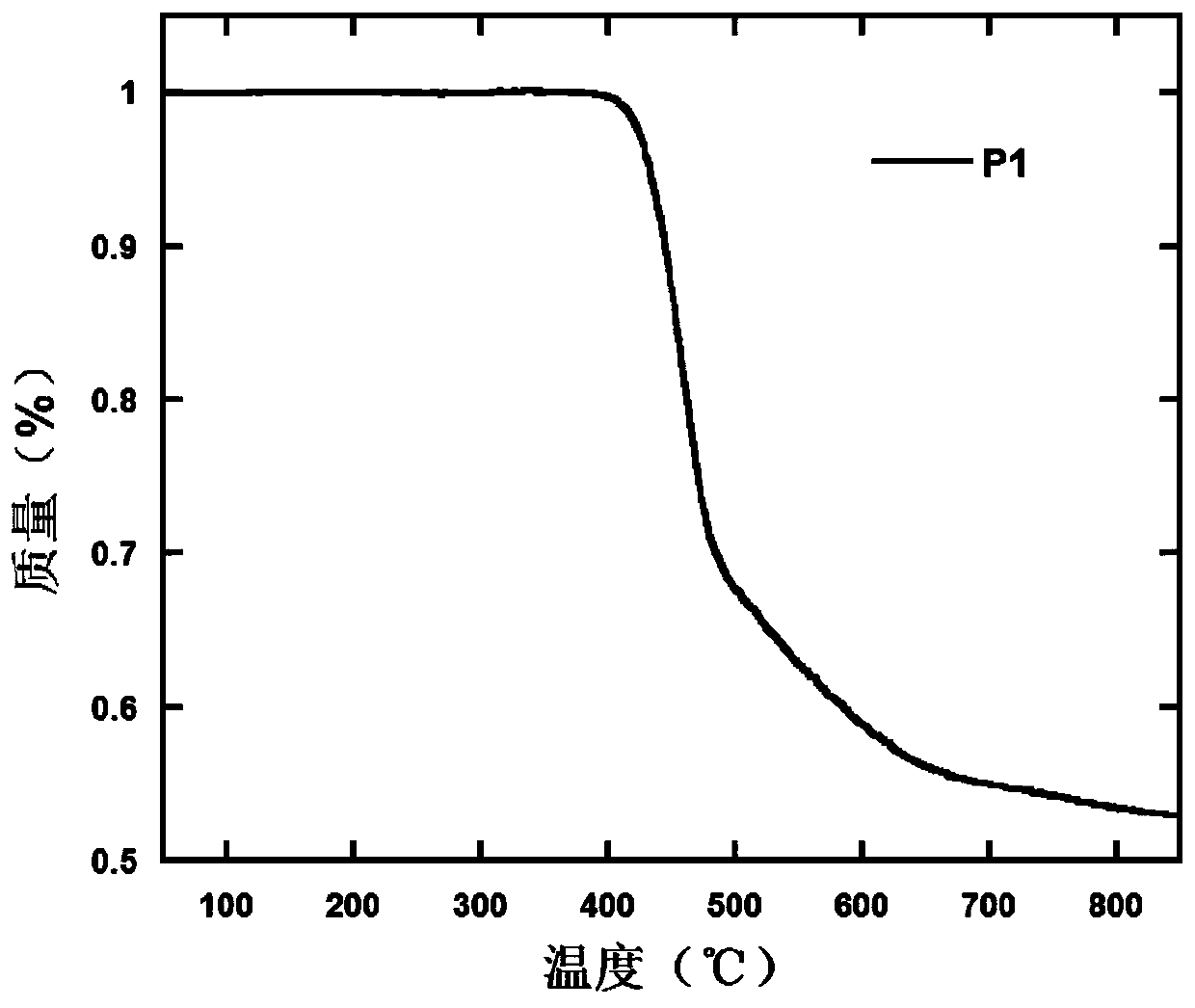
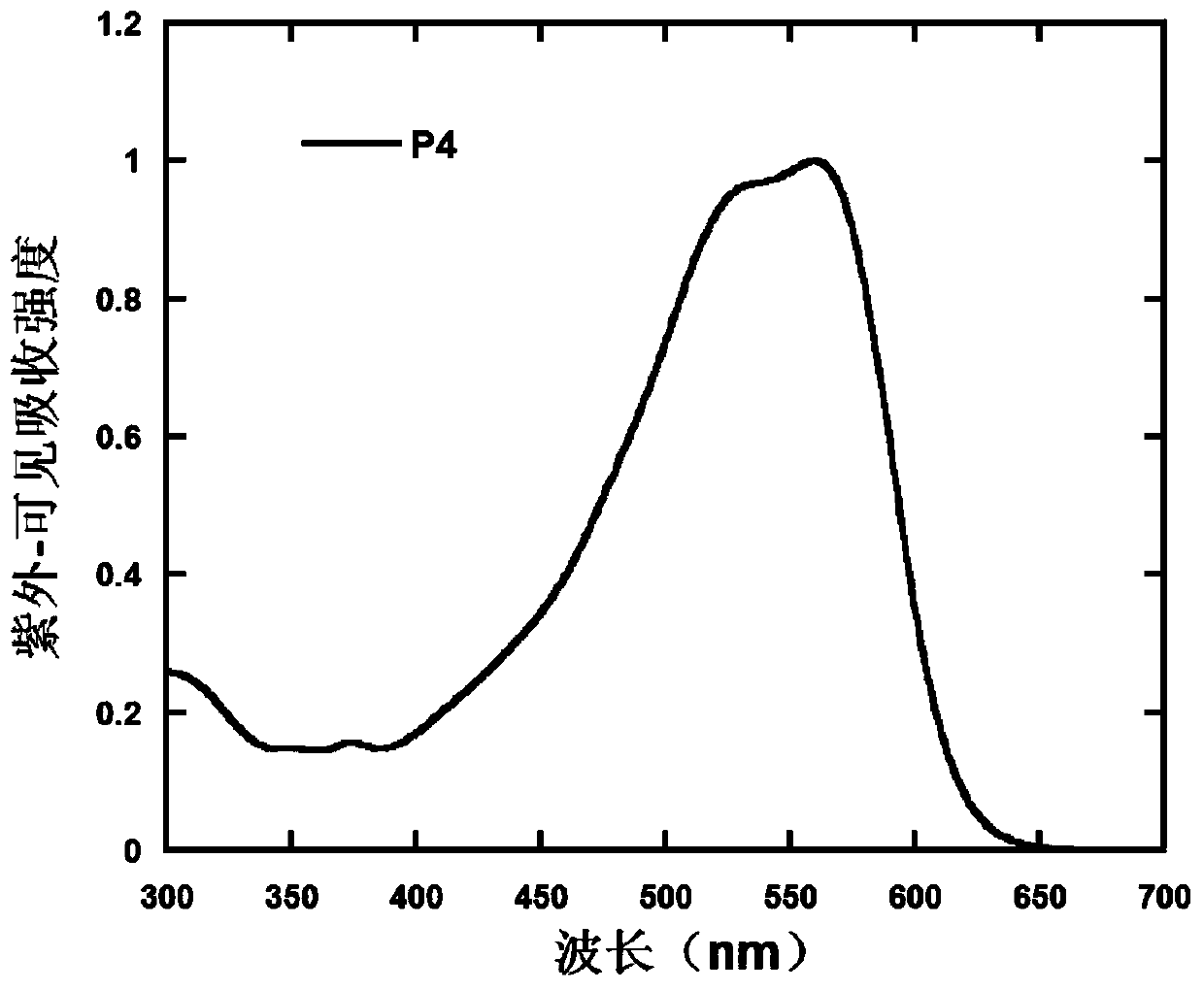
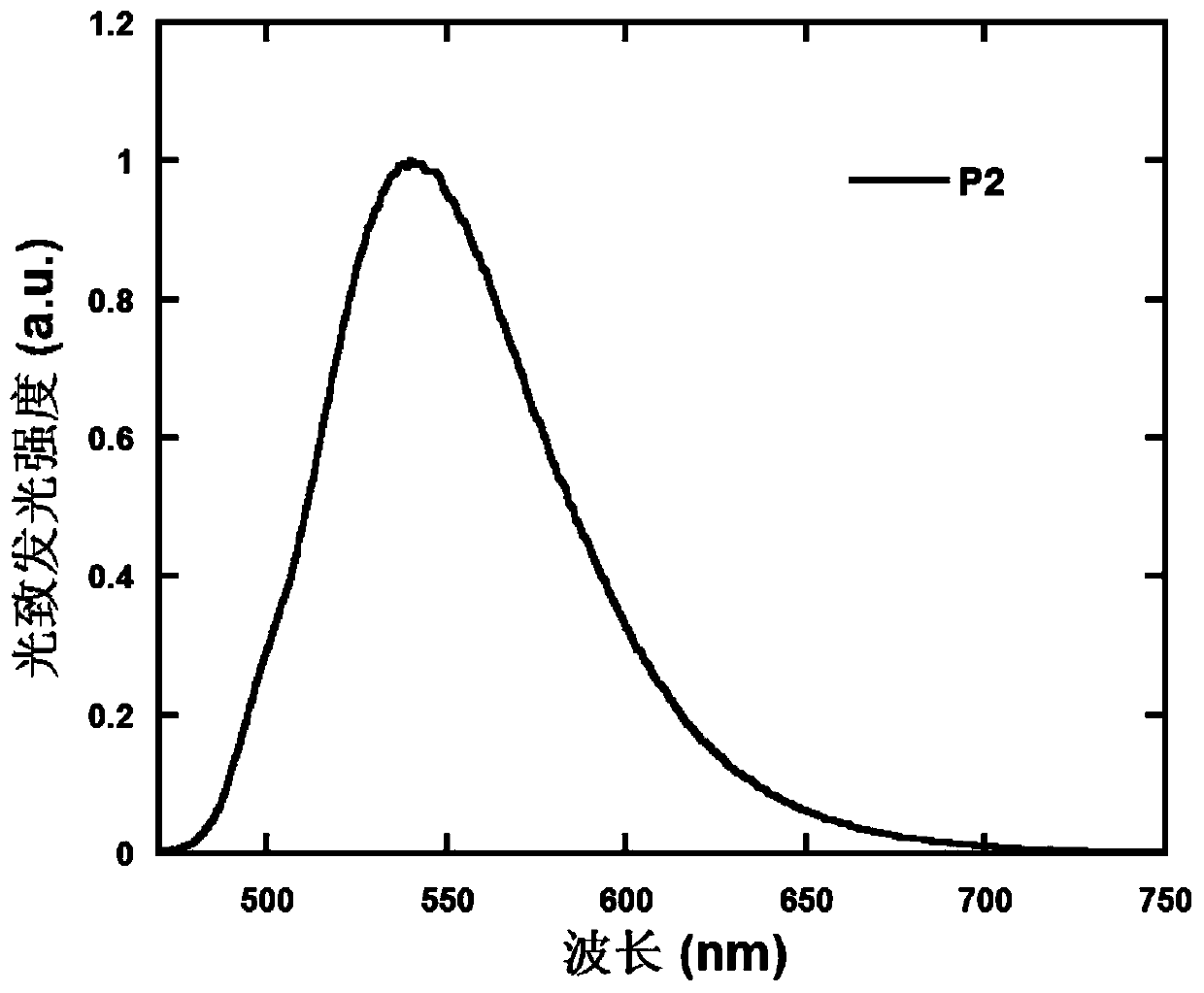
Polymer containing 9,9,10,10-tetraoxo-thianthracene five-membered condensed ring unit and its preparation method and application
A technology of polymer and polymerization reaction, applied in the direction of electrical components, semiconductor/solid-state device manufacturing, electric solid-state devices, etc., can solve problems such as reducing LUMO energy level, and achieve the effect of improving electroluminescent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Preparation of Compounds 4 and 5
[0064] (1) Preparation of compound 1
[0065] Under nitrogen protection, 2-bromo-7-iodo-9,9-dimethylfluorene (3.99g, 10mmol), 4-bromo-1,2-benzenedithiol (2.21g, 10mmol ), sodium tert-butoxide (4.81g, 50mmol), tris(dibenzylideneacetone)dipalladium (458mg, 0.5mmol), bis(2-diphenylphosphophenyl)ether (269mg, 0.5mmol) and 120mL toluene, heated to 50°C and stirred for 6 hours. After the reaction was completed, the product was extracted with dichloromethane, the organic phase was washed with saturated aqueous sodium chloride solution, the solvent was evaporated under reduced pressure, and the crude product was eluted with a mixed solvent of petroleum ether:dichloromethane=6:1 (v / v) Purified by solvent column chromatography to obtain 3.11 g of white solid with a yield of 63%. 1 H NMR, 13 The results of CNMR, MS and elemental analysis showed that the obtained compound was the target product.
[0066] (2) Preparation of compound 2
[0067...
Embodiment 2
[0075] Preparation of Compound M1
[0076] Compound 4 (4.90g, 10mmol) and 150mL tetrahydrofuran and acetic acid mixed solvent (v:v=1:1) were added to a 300mL two-necked flask, and aqueous hydrogen peroxide solution (10mL, 100mmol) was added dropwise, and the reaction was stirred for 12 hours. After the reaction was completed, the product was extracted with dichloromethane, washed with saturated aqueous sodium chloride, and the solvent was removed under reduced pressure. The crude product was used as the eluent column layer with a mixed solvent of petroleum ether:dichloromethane=3:1 (v / v) Analysis and purification gave 4.44 g of white solid with a yield of 80%. 1 H NMR, 13 C NMR, MS and elemental analysis results show that the compound obtained is the target product, and its chemical reaction equation is as follows:
[0077]
Embodiment 3
[0079] Preparation of compound M2
[0080] Add compound 5 (4.90g, 10mmol) and 150mL tetrahydrofuran and acetic acid mixed solvent (v:v=1:1) into a 300mL two-necked flask, slowly add aqueous hydrogen peroxide solution (10mL, 100mmol), and stir for 12 hours. After the reaction was completed, the product was extracted with dichloromethane, washed with saturated aqueous sodium chloride, and the solvent was removed under reduced pressure. The crude product was used as the eluent column layer with a mixed solvent of petroleum ether:dichloromethane=3:1 (v / v) Analysis and purification gave 4.72 g of white solid with a yield of 85%. 1 H NMR, 13 C NMR, MS and elemental analysis results show that the compound obtained is the target product, and its chemical reaction equation is as follows:
[0081]
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com