Preparation method of Roxadustat
A technology for biopharmaceuticals and compounds, which is applied in the field of preparation of biopharmaceuticals, can solve the problems of unfavorable industrial production and high requirements for reaction temperature equipment, and achieve the effects of good industrialization prospects, reduction of by-products, and simplified process routes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
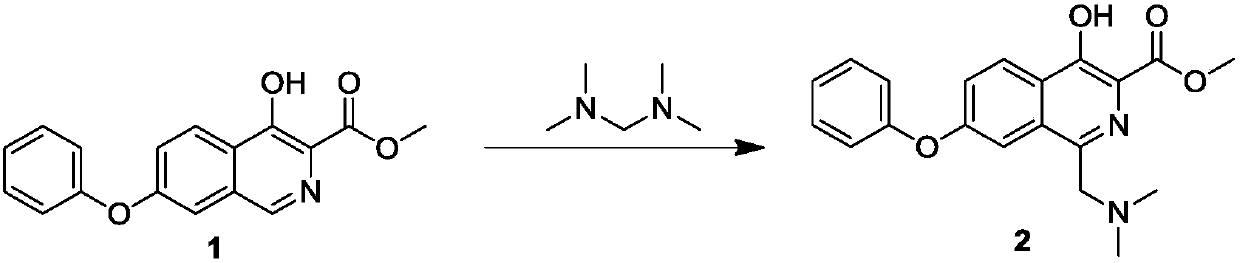
[0028] Preparation of compound 2:
[0029]
[0030] Compound 1 (100g, 0.338mol) was dissolved in 215mL acetic acid / trifluoroacetic acid (volume ratio: 7 / 1) mixed solvent, and tetramethylaminomethane (43.25g, 0.423mol) was slowly added dropwise. After the addition was complete, the temperature was raised to React at 45°C for 15 hours, the TLC plate detects that the reaction is complete, the reaction solution is cooled to room temperature, and directly put into the next step without any treatment.
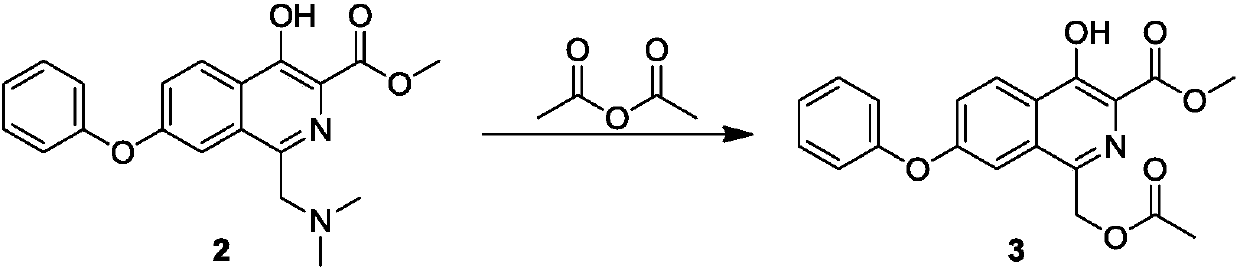
[0031] Preparation of compound 3:
[0032]
[0033] At room temperature, acetic anhydride (43.13 g, 0.423 mol) was added dropwise to the reaction solution for the preparation of Compound 2 above. After the addition was complete, the reaction solution was heated to 90° C. for 12 hours. The TLC plate detected that the reaction was complete, and the reaction solution was cooled to room temperature and added dropwise to 1L of water, the solid product slowly precipitated, filtered ...
Embodiment 2
[0041] Preparation of compound 2:
[0042]
[0043] Compound 1 (150g, 0.507mol) was dissolved in 300mL acetic acid / trifluoroacetic acid (volume ratio: 10 / 1) mixed solvent, and tetramethylaminomethane (64.88g, 0.634mol) was slowly added dropwise. After the addition was complete, the temperature was raised to React at 50°C for 12 hours, the TLC plate detects that the reaction is complete, the reaction solution is cooled to room temperature, and directly put into the next step without any treatment.
[0044] Compound 3 was prepared according to the method described in Example 1 to obtain 155 g of Compound 3 with a total yield of 83.07% and an HPLC purity of 99.05%.
[0045] Preparation of compound 4:
[0046]
[0047]Add compound 3 (150g, 0.41mol), 750ml ethyl acetate, sodium carbonate (24.2g, 0.22mol), Pd / C (22.5g) in sequence to the hydrogenation reactor, and pressurize the hydrogen to 0.8MPa after nitrogen replacement , the reaction solution was heated to 50°C for 12 h...
Embodiment 3
[0052] Compound 2, Compound 3 and Compound 4 were prepared as described in Example 2.
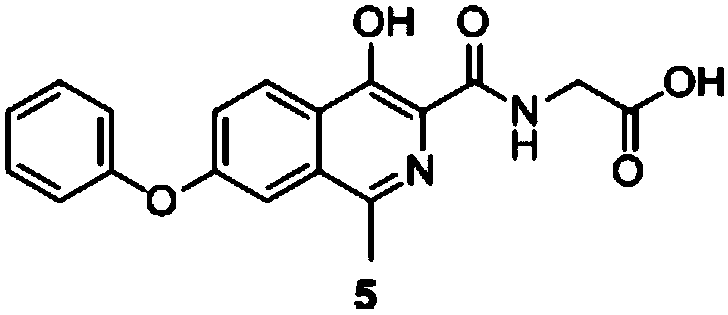
[0053] Preparation of Compound 5:
[0054]
[0055] Compound 4 (30g, 0.097mol) and glycine (36.4g, 0.485mol) were dissolved in ethylene glycol methyl ether (150mL), then triethylamine (TEA) (29.45g, 0.291mol) was added, and the mixture was heated to React at 95°C for 1.5h, TLC plate detects that the reaction is complete, the reaction solution is lowered to room temperature to concentrate the solvent, add 300mL of water to dissolve, extract with ethyl acetate three times, collect the aqueous phase and adjust the pH with dilute hydrochloric acid, the solid slowly precipitates, filter, filter cake After washing with water and then with acetone, the filter cake was collected and dried to obtain 31.8 g of compound 5, with a total yield of 92.98% and an HPLC purity of 99.79%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com