A method for the simultaneous quantitative determination of phospholipids and fatty acid glycerides in pharmaceutical preparations
A technology of fatty acid glycerides and pharmaceutical preparations, applied in measuring devices, instruments, scientific instruments, etc., can solve the problems of insufficient sensitivity of high-performance liquid phase analysis methods, consumption of large organic solvents such as chloroform, and non-compliance with environmental protection, etc., to avoid Effects of mutual interference and interference of other components, less amount of organic solvent, and short detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The pretreatment of embodiment one sample
[0026] Measure 1.0mL of the drug preparation sample into a 20mL headspace bottle, place it in a vacuum drying oven at 65°C and dry it under reduced pressure until dry, add 1.0mL of internal standard solution and 0.5mL of 1mol / L potassium hydroxide solution, seal it, and keep at 90°C Heat in a water bath and vibrate continuously for 10 minutes, cool to room temperature to obtain a saponified sample solution; add 10% boron trifluoride methanol solution 1mL to the saponified sample solution, seal, heat in a 90°C water bath and vibrate continuously After 5 minutes, cool to room temperature to obtain the sample solution after methyl esterification treatment; add 5 mL of n-hexane to the sample solution after methyl esterification treatment, seal, shake in a water bath at 60°C for 3 minutes, cool to room temperature, add 5 mL of saturated sodium chloride, Seal it, shake it at room temperature for 2 minutes, let it stand for stratific...
Embodiment 2
[0028] The preparation of embodiment two reference substance solution and negative solution
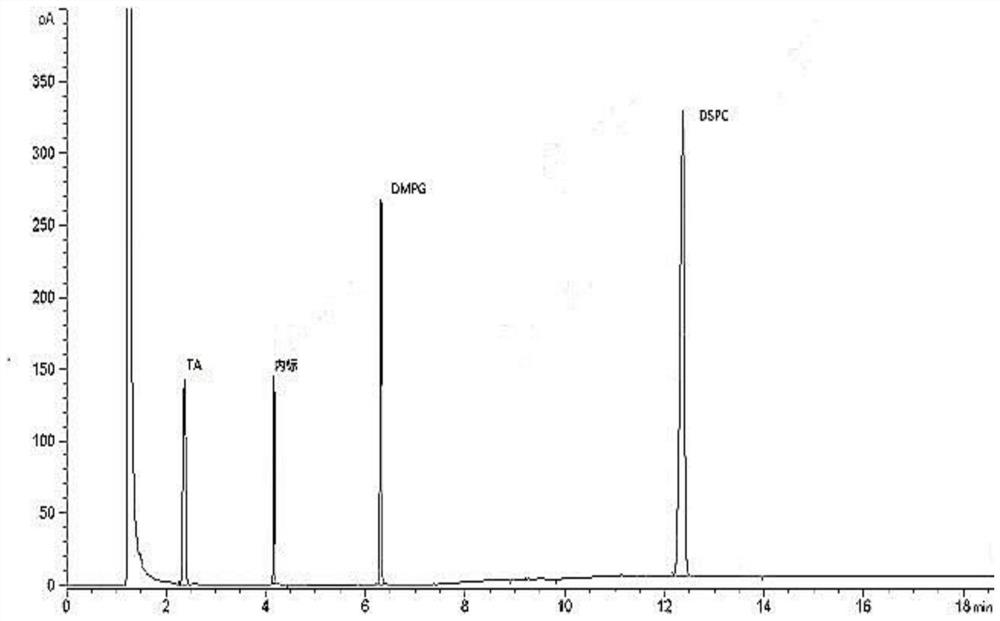
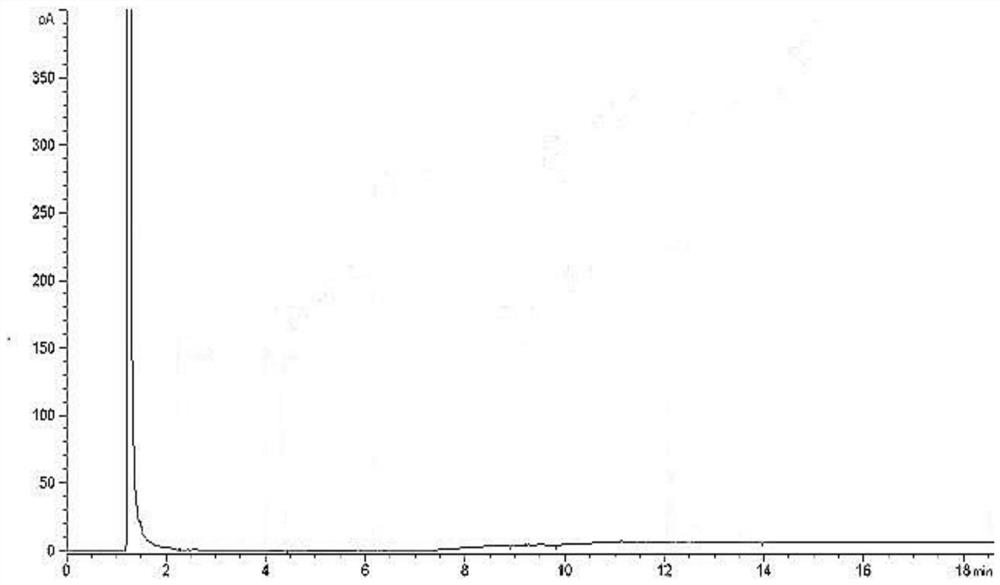
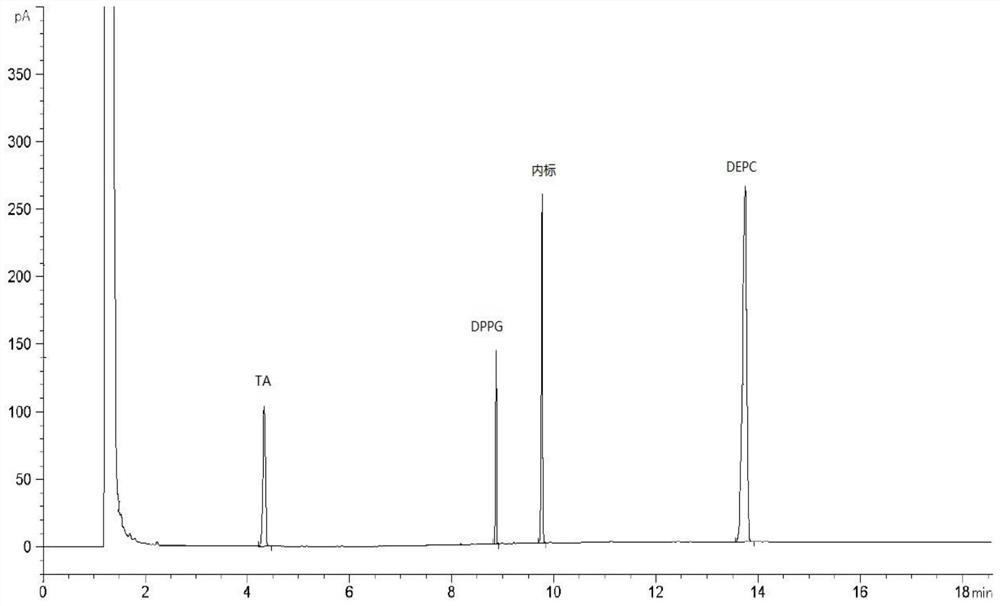
[0029] Take 15.00mg TA, 20.00mg DMPG and 60.00mg DSPC respectively, accurately weigh them into a 10mL volumetric flask, and dilute to volume with methanol to obtain a stock solution. Measure 1.0 mL of the stock solution in a headspace bottle, and prepare according to the method in Example 1 to obtain the reference substance solution. Take 1.0 mL of pure water in a headspace bottle and prepare according to the method in Example 1 to obtain a negative solution.
Embodiment 3
[0030] Example three detection of sample detection solution
[0031] The sample detection solution prepared in Example 1, the reference substance solution prepared in Example 2 and the negative solution are detected by gas chromatography, and the gas chromatography conditions include: the chromatographic column adopts a medium polar chromatographic column DB-WAX, and the carrier gas Helium, flow rate of 2mL / min, hydrogen flow rate of 30mL / min, air flow rate of 300mL / min, inlet temperature of 250°C, injection volume of 1μL, split injection, split ratio of 10:1, detection The detector is a hydrogen flame ionization detector, the detector temperature is 260°C, and the temperature is programmed: the initial temperature is 80°C, kept for 5 minutes, and the temperature is raised to 230°C at 50°C / min, and kept for 11 minutes. Record the chromatogram see figure 1 , the chromatographic peaks of TA, DMPG, DSPC and the internal standard in the sample detection liquid chromatogram are co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com