A novel metal ion tolerance keratinase and its application
A keratinase and nucleotide sequence technology, applied in the field of industrial biology, can solve the problems of unfavorable wide application and limited application of nano-silver, and achieve the effects of good tolerance, good bacteriostatic activity and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] This example illustrates a method for screening keratinase genes based on metagenomic technology.
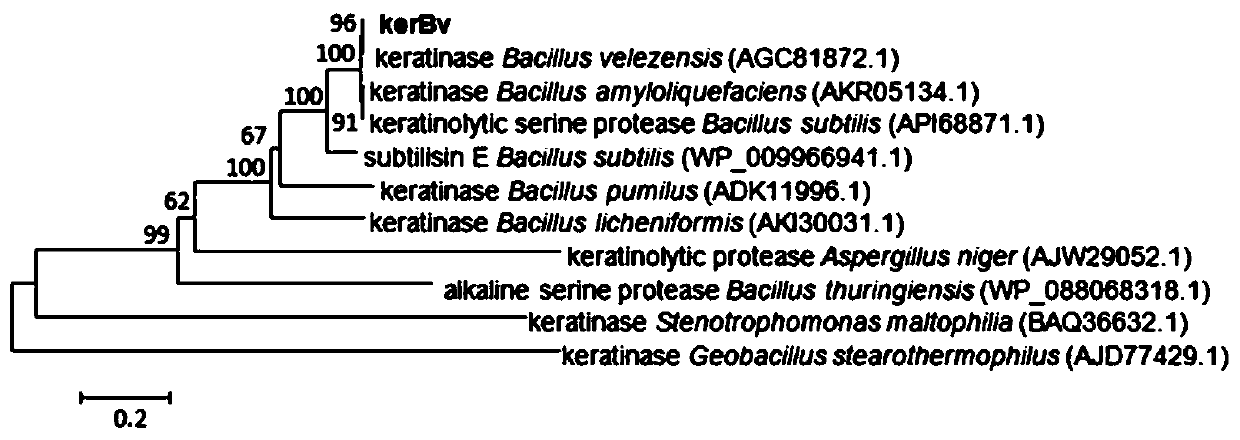
[0043] Soil samples were collected from the place where furs were piled up in the tannery, and the total DNA of the samples was extracted with a soil genome extraction kit. According to the conserved sequence of keratinase, degenerate primers were designed for PCR amplification reaction, the amplified fragments were sequenced and compared with the NCBI database, and then primers were further designed according to the results, and the complete keratinase gene coding sequence was amplified. After the obtained keratinase sequence was analyzed by phylogenetic tree, it was found that it was most similar to the sequence of keratinase derived from Bacillus velezensis ( figure 1 ). This method is based on metagenomic technology, combined with the conserved sequence of keratinase in the database for direct amplification and screening. Compared with the screening method based on ...
Embodiment 2
[0045] This example illustrates the construction process of the keratinase Bacillus subtilis expression system.
[0046] According to the keratinase gene sequence, primers with restriction sites were designed, and the keratinase coding gene sequence was obtained by PCR amplification:
[0047] Upstream primer: 5'-CG GGATCC ATGAGAGGCAAAAAGGTATGG-3'
[0048] Downstream primer: 5'-CG ACGCGT TTACTGAGCTGCCGCCTGTA-3'
[0049] The PCR amplification reaction was carried out in a 40 μL system, and 20 μL PrimeSTAR HS, 17 μL ddH 2 O, 1 μL of template DNA, 1 μL of upstream and downstream primers. The reaction conditions were as follows: 30 cycles of denaturation at 95°C for 5 minutes, denaturation at 95°C for 30 seconds, annealing at 50°C for 30 seconds, extension at 72°C for 1 minute, and a total of 35 cycles; final extension at 72°C for 10 minutes.
[0050] The PCR product was identified by nucleic acid electrophoresis, recovered and purified by tapping gel, the recovered product w...
Embodiment 3
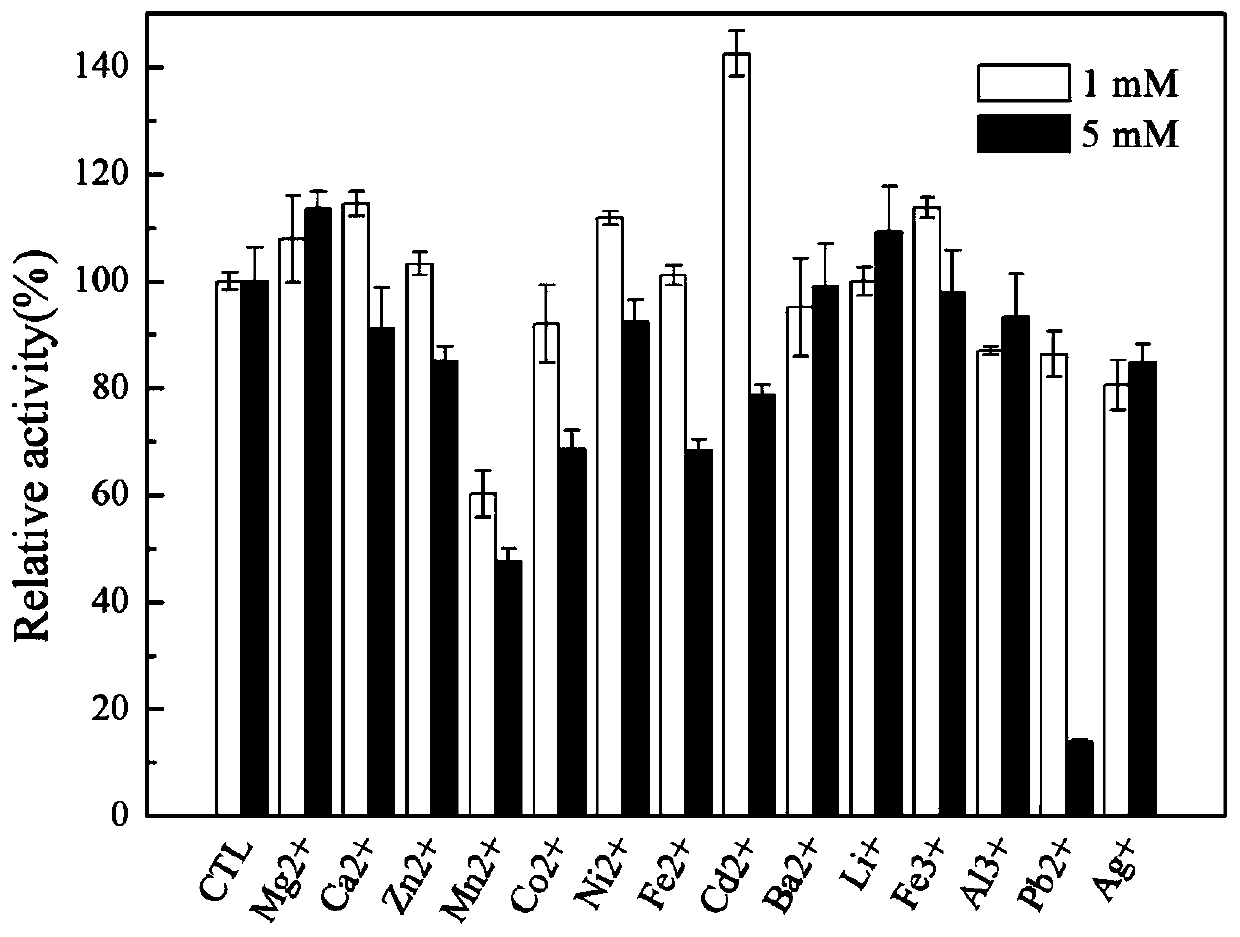
[0052] This example illustrates the detection method of recombinant keratinase enzyme activity.
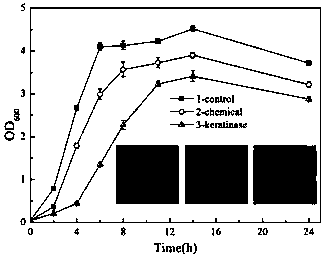
[0053] Preparation of 1% keratin substrate: first prepare a 0.1M Tris-HCl (pH=9.0) solution, then add 5% soluble keratin mother liquor, then add deionized water to dilute the Tris-HCl solution concentration to 0.05M, And the keratin solution concentration was diluted to 1%. Enzyme reaction: Take 100 μL of moderately diluted enzyme solution, add 0.1 mL of substrate solution, place in a 50°C water bath for accurate reaction for 15 minutes, and immediately add 200 μL of 5% TCA to terminate the reaction. In the control group, 200 μL of TCA was added first, and then 0.1 mL of substrate solution was added after the enzyme reaction was completed. After the above reaction, centrifuge at 12000rpm for 5min, pipette 200μL supernatant into a new 1.5mL EP tube, add 1mL 0.4M Na 2 CO 3 , and then added 200 μL of folinol, placed in a water bath at 40°C for 20 minutes for color reaction, and th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com