Preparation method of polyethylene glycol modified nano-micelle or vesicle with pH (potential of hydrogen) responsivity and high drug loading capacity
A polyethylene glycol and high drug-loading technology, which is applied in the fields of polymer materials and biomedical engineering, can solve the problems of no targeting effect and large lethality of normal cells, and achieve increased drug loading, improved release, The effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
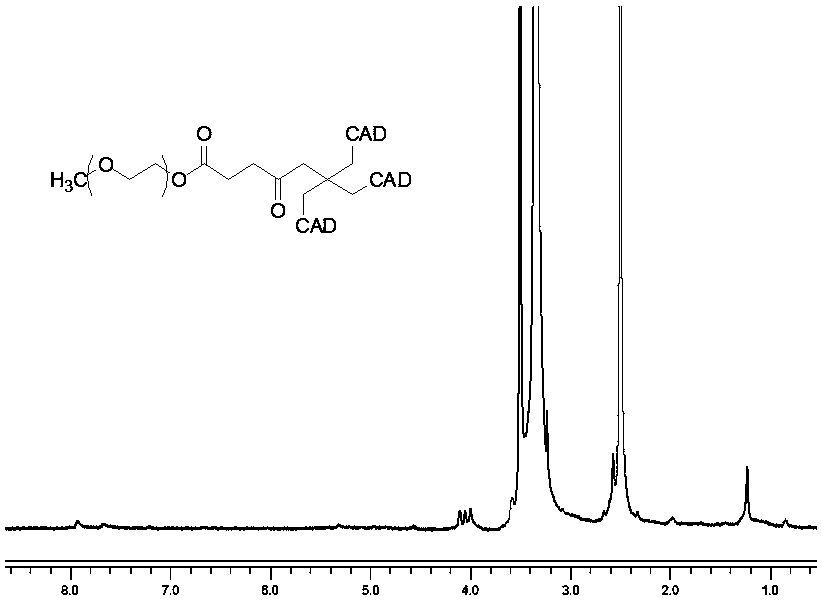
[0025] Dissolve 0.75 g of polyethylene glycol monomethyl ether with a molecular weight of 750 and 0.12 g of succinic anhydride in 30 ml of tetrahydrofuran, reflux at 75°C for 12 hours, precipitate with ether for 3 times, filter, and vacuum-dry the filter cake to constant weight to obtain carboxyl-terminated Modified polyethylene glycol: 34.0 mg of pentaerythritol, 0.13 g of 4-dimethylaminopyridine and 0.61 g of dicyclohexylcarbodiimide were dissolved in 10 ml of dichloromethane to form a pentaerythritol solution, and the carboxyl-modified polyethylene glycol Dissolve 0.75g of alcohol in 10ml of dichloromethane, add it dropwise to the pentaerythritol solution at a very slow speed, stir thoroughly for 2 days, precipitate with ether for 3 times, filter, and vacuum dry the filter cake to obtain a trihydroxy-modified polyethylene glycol Alcohol; 50 mg of doxorubicin hydrochloride was dissolved in 10 ml of deionized water to form an aqueous solution, 50 mg of cis-aconitic anhydride w...
Embodiment 2
[0028]Dissolve 1.0 g of polyethylene glycol monomethyl ether with a molecular weight of 1000 and 0.16 g of succinic anhydride in 30 ml of tetrahydrofuran, reflux at 75°C for 12 hours, precipitate with methanol for 3 times, filter, and vacuum-dry the filter cake to constant weight to obtain carboxyl-terminated Modified polyethylene glycol: 34.0 mg of pentaerythritol, 0.13 g of 4-dimethylaminopyridine and 0.61 g of dicyclohexylcarbodiimide were dissolved in 10 ml of chloroform to form a pentaerythritol solution, and the carboxyl-modified polyethylene glycol Dissolve 1.0 g of alcohol in 10 ml of chloroform, add it dropwise to the pentaerythritol solution at a very slow speed, stir thoroughly for 2 days, precipitate with ether for 3 times, filter, and vacuum dry the filter cake to obtain a trihydroxy-modified polyethylene glycol alcohol; 50.0 mg of doxorubicin hydrochloride was dissolved in 10 ml deionized water to form an aqueous solution, 50.0 mg of cis-aconitic anhydride was dis...
Embodiment 3
[0031] Dissolve 2.0 g of polyethylene glycol monomethyl ether with a molecular weight of 2000 and 0.16 g of succinic anhydride in 30 ml of tetrahydrofuran, reflux at 75°C for 12 hours, precipitate with n-hexane for 3 times, filter, and vacuum-dry the filter cake to constant weight to obtain terminal Carboxy-modified polyethylene glycol; 34.0 mg of pentaerythritol, 0.13 g of 4-dimethylaminopyridine and 0.61 g of dicyclohexylcarbodiimide are dissolved in 10 ml of dimethylformamide to form a pentaerythritol solution. Dissolve 0.75 g of polyethylene glycol in 10 ml of dimethylformamide, add dropwise to the pentaerythritol solution at a very slow speed, stir thoroughly for 2 days, precipitate with n-hexane for 3 times, filter, and vacuum-dry the filter cake to obtain a constant weight. Hydroxy-modified polyethylene glycol; 50.0 mg of doxorubicin hydrochloride was dissolved in 10 ml of deionized water to make an aqueous solution, 50.0 mg of cis-aconitic anhydride was dissolved in 10 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com