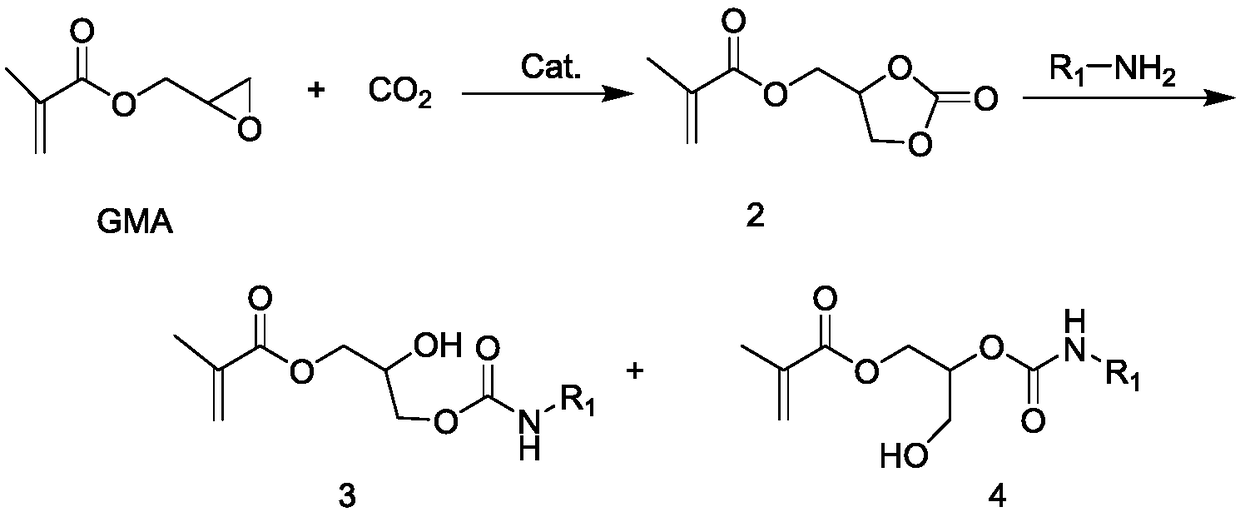
Urethane compound and synthesis and application thereof
An ester compound and compound technology, which is applied in the preparation of organic compounds, the preparation of carbamate derivatives, organic chemistry, etc., can solve problems such as unsatisfactory mechanical properties, and achieve the goal of being suitable for large-scale production, the preparation method is simple and the reaction process is simple. Highly active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The synthetic route of the preparation method of the glycidyl methacrylate urethane compound is shown in Figure 1, and the synthetic process may include the following steps:
[0042] (1) Glycidyl methacrylate and phase transfer catalyst are added to the reactor, and then CO 2 Carry out chemical reaction, obtain glycidyl methacrylate base cyclic carbonate intermediate after reaction finishes;
[0043] (2) Then, the glycidyl methacrylate-based cyclic carbonate intermediate obtained in step (1) is dissolved and then reacted with an amine compound to obtain a glycidyl methacrylate-based urethane compound.
[0044] The phase transfer catalyst described in the step (1) is a quaternary ammonium salt (formula (3)) or a quaternary phosphonium salt (formula (4)). The reaction temperature is between 80 and 120 °C, and the reaction pressure is preferably between 2 and 6 MPa (that is, CO is introduced into the reactor before the reaction 2 A high-pressure environment of 2-6 MPa is...
Embodiment 1
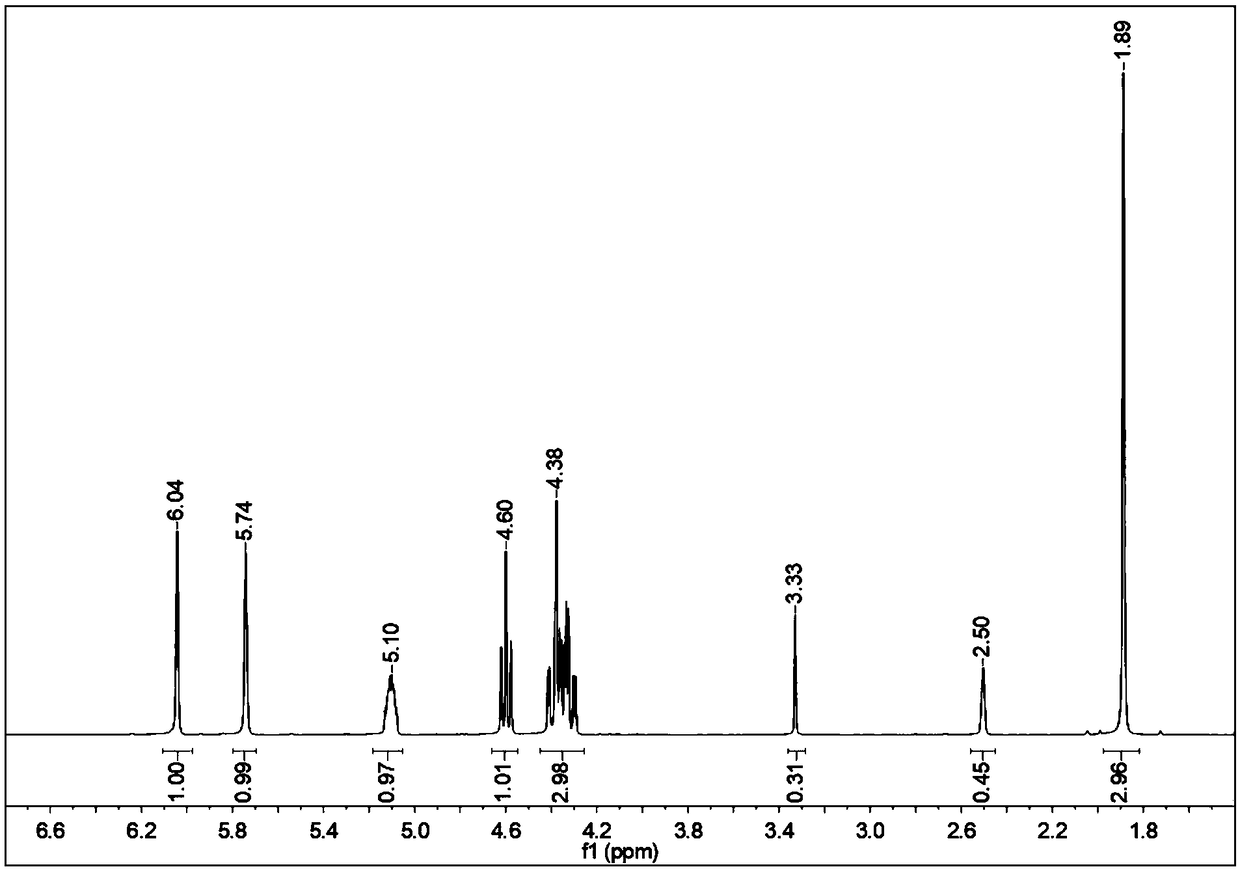
[0055] Add 200mmol of glycidyl methacrylate and 10mmol of tetrabutylammonium bromide into the autoclave, and fill the autoclave with CO 2 , so that the pressure of the autoclave is 6.0 MPa. Then the autoclave is placed in a constant temperature oil bath of 120° C. After 4 hours of reaction, the pressure in the autoclave no longer decreases, and the reaction is stopped (of course, the present invention can also directly use time as a judgment indicator for the termination of the reaction, even if the pressure is still is decreasing). After the reaction, the autoclave was cooled to room temperature, and then the carbon dioxide was slowly removed. The crude product obtained by the reaction was separated and purified by column chromatography, and the catalyst tetrabutylammonium bromide was removed by using dichloromethane as an eluent to obtain a glycidyl methacrylate-based cyclic carbonate. That 1 H-NMR analysis results show (as attached figure 2 Shown), the conversion rate ...
Embodiment 2
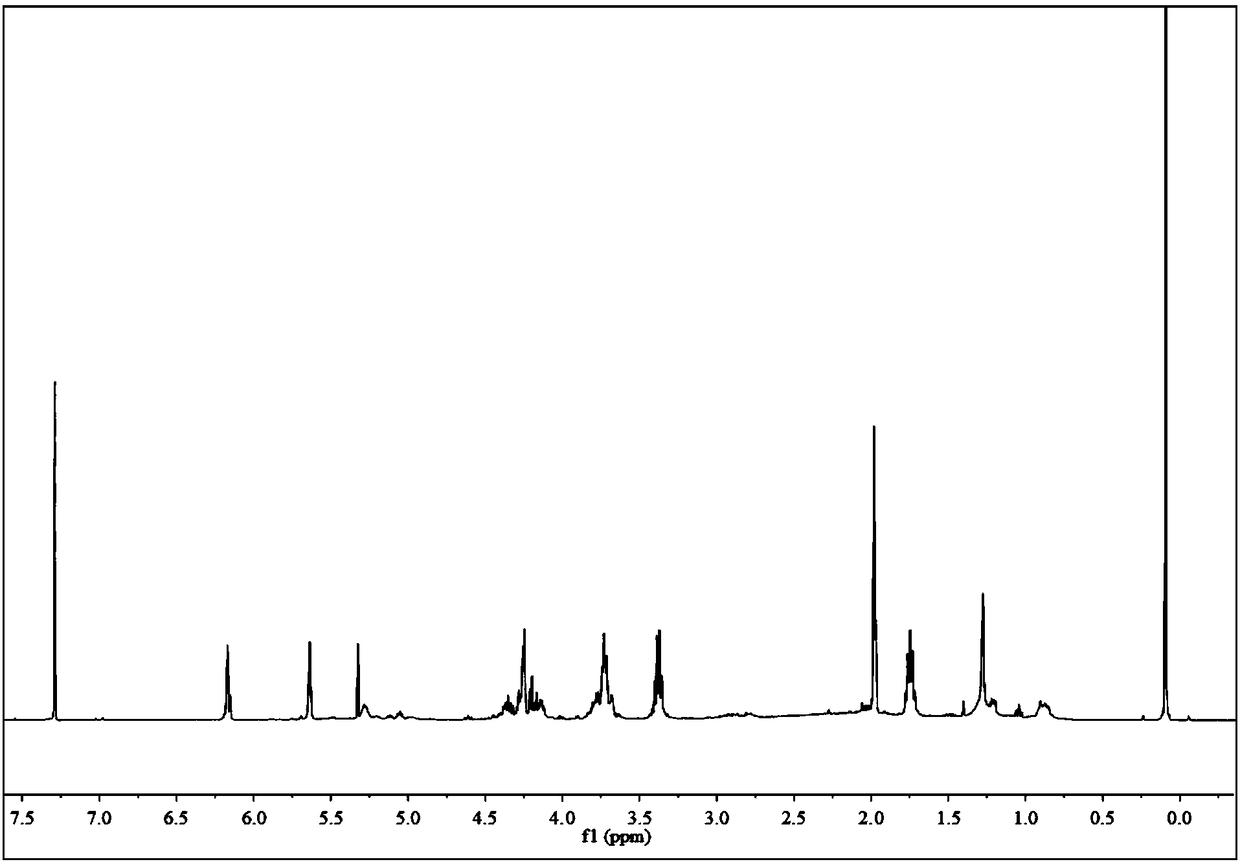
[0059] Add 200mmol of glycidyl methacrylate and 6mmol of tetraethylammonium nitrate into the autoclave, and fill the autoclave with CO 2 , the pressure of the autoclave was raised to 6.0MPa, and then the autoclave was placed in a constant temperature oil bath of 80°C. After 18 hours of reaction, the pressure in the autoclave no longer decreased, and the reaction was stopped. Then the autoclave was cooled to room temperature, and carbon dioxide was slowly removed. The crude product obtained by the reaction was separated and purified by column chromatography, and the catalyst tetraethylammonium nitrate was removed by using dichloromethane as an eluent to obtain glycidyl methacrylate-based cyclic carbonate.
[0060] Add 100 mmol of glycidyl methacrylate-based cyclic carbonate synthesized above and 100 mmol of 2-amino-1-ethanol into the reactor, and add 20 mL of dichloromethane as a solvent into the reactor at the same time, at 20 ° C, nitrogen The reaction was carried out under ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com