Microchannel reactor-based cyano hydrolysis reaction method
A technology of microchannel reactor and hydrolysis reaction, applied in the direction of chemical instruments and methods, chemical/physical/physical chemical reactors, chemical/physical/physical chemical fixed reactors, etc., can solve the problem of difficult control of reactions and product purity Insufficient, low hydrolysis efficiency and other problems, to achieve the effect of increasing the hydrolysis rate, controlling the reaction time, and shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
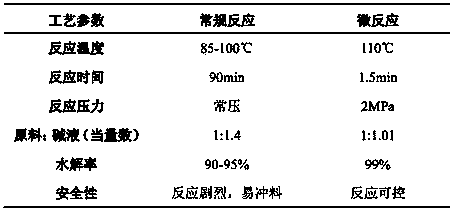
[0037] Preparation of β-alanine: 80% aminopropionitrile (technical grade) aqueous solution and 30% sodium hydroxide aqueous solution are used as raw materials.
[0038] Process operation steps:
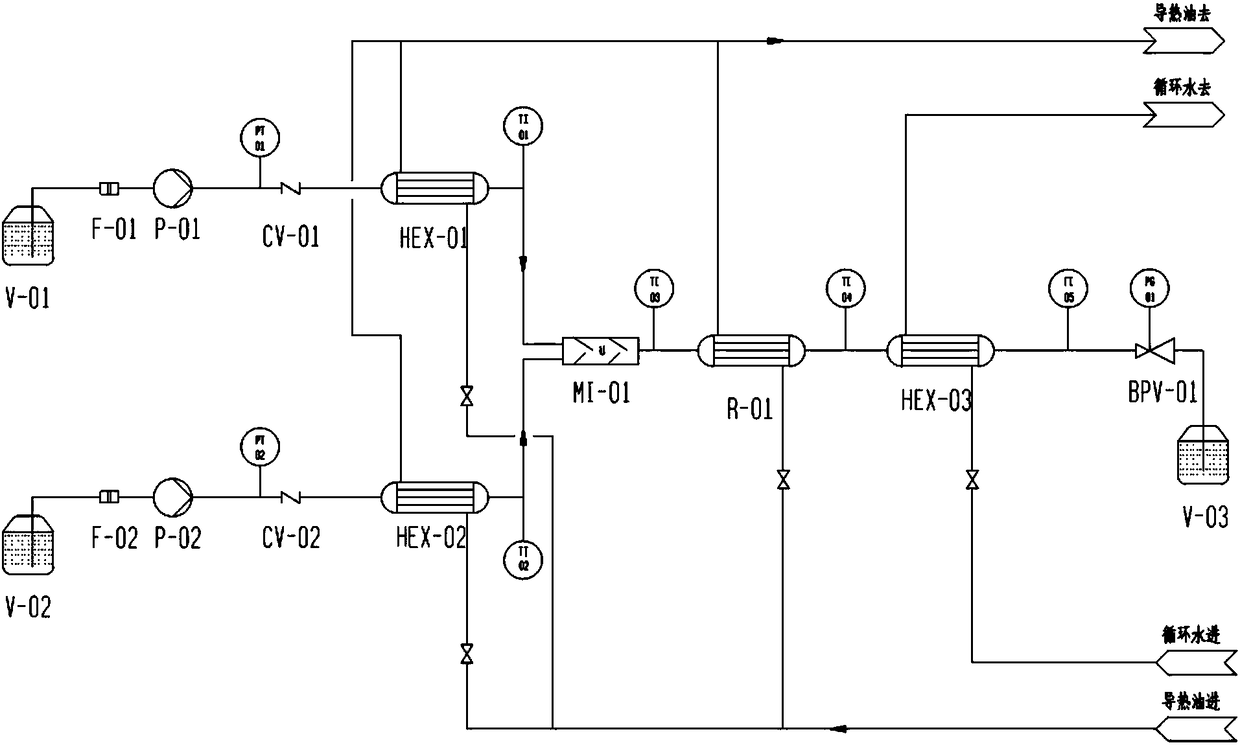
[0039] 1) Connection of micro-reaction equipment: connect all equipment such as figure 1 Connection shown, using Starlam3000 microreactor;
[0040] 2) Debugging of micro-reaction equipment: Turn on the constant temperature circulator, set the temperature at 110°C, inject water into the micro-reaction equipment through a constant-flow pump, clean the internal pipeline of the micro-reaction equipment for 10 minutes, and open the back pressure valve at the same time to make the system back pressure 5.0MPa , Check whether the equipment has liquid leakage or air leakage, whether the temperature and pressure monitoring equipment are accurate, and proceed to the next step after everything is correct;
[0041] 3) Flow correction of constant flow pump: constant flow pump P01 and P02 use wate...
Embodiment 2
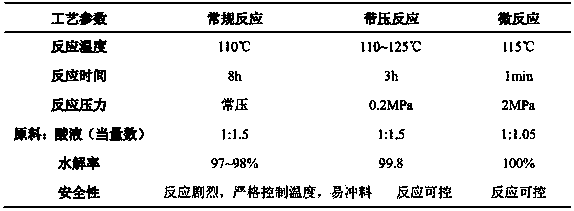
[0050] Preparation of 3-mercaptopropionic acid: use 95% 3-mercaptopropionitrile and 20% hydrochloric acid solution as raw materials without using solvent.
[0051] Process operation steps:
[0052] 1) The microreactor system is connected, and the debugging is the same as the above operation;
[0053] 2) Preparation: 5g of 3‐mercaptopropionitrile with a content of 95% is stored in storage tank V‐01, fed by pump P‐01; 10.92 g of 20% hydrochloric acid solution is placed in storage tank V‐02 The storage is fed by the pump P-02, the inlet temperature of the microreactor for the hydrochloric acid solution is 110°C, the outlet temperature of the microreactor is 114°C, and after an equilibrium time of 5 minutes, the reaction temperature rises to 115°C, and the feeding starts;
[0054] 3) Mix in the micro-mixer U, and complete the hydrolysis reaction through a tube reactor and a heat exchanger within 1 minute;
[0055] 4) The material after the reaction is completed enters the receiv...
Embodiment 3
[0060] Preparation of 7Α-double-condensed pyrrolidine-acetic acid: Pisicani intermediate 7A-double-condensed pyrrolidine-acetonitrile, 36% hydrochloric acid solution as raw material.
[0061] Process operation steps:
[0062] 1) The microreactor system is connected, and the debugging is the same as the above operation;
[0063] 2) Preparation: 10 g of Pisicanilic intermediate 7A-double-condensed pyrrolidine-acetonitrile with a content of 95% is stored in the storage tank V-01 and fed by the pump P-01; the 36% hydrochloric acid solution 14.13 g is placed in the storage tank V-02 and fed by the pump P-02. The inlet temperature of the microreactor of the hydrochloric acid solution is 100°C, and the outlet temperature of the microreactor is 103°C. After the equilibrium time is 5 minutes, the reaction temperature rises to 105°C , start receiving materials;
[0064] 3) Mix in the micro-mixer U, and complete the hydrolysis reaction in 3 minutes through the tube reactor and heat exc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com