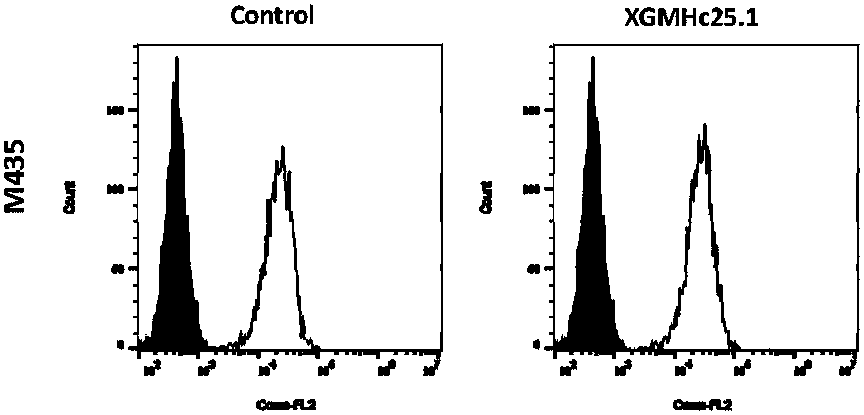
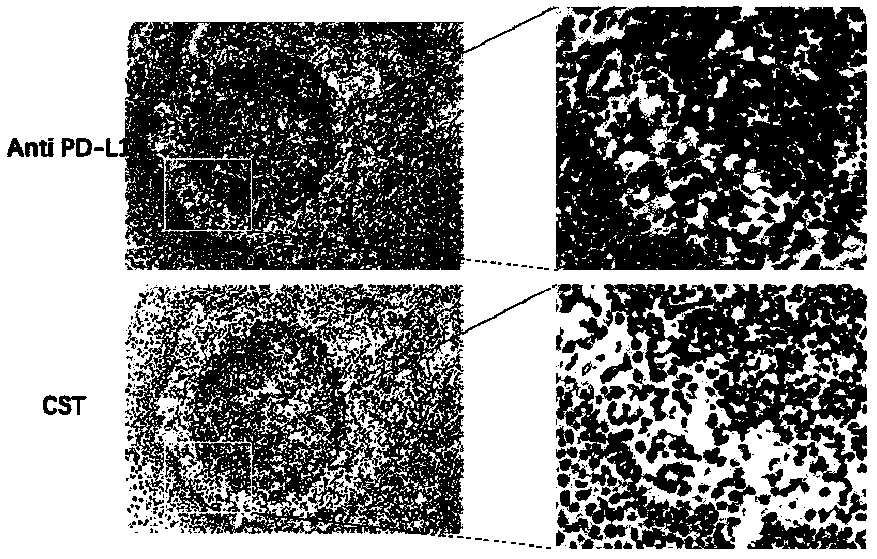
Anti-human B7-H1 monoclonal antibody preparation method and application of antibody to immunohistochemical detection
A B7-H1, monoclonal antibody technology, applied in the direction of anti-animal/human immunoglobulin, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, immunoglobulin, etc., to improve the diagnostic accuracy , the effect of improving the accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Obtaining of Hybridomas Producing B7-H1 Monoclonal Antibody
[0046] (1) Immune mice
[0047] Mice were immunized with fusion protein or transgenic cells, immunized four times with an interval of 21 days each time, and the orbital blood titer of the mice was measured 7-10 days after the fourth immunization.
[0048] (2) Cell culture
[0049] 1. One day before fusion, take one BALB / c mouse aged 6-7 weeks and place it in 75% ethanol solution for 2 minutes.
[0050] 2. The mouse spleen was aseptically taken out, placed in a 200-mesh stainless steel screen, and ground to obtain a single cell suspension. Wash twice with 1640 basal medium (1400rpm, 5min) for later use. Use 15% FBS 1640 medium to adjust the cell concentration to 2×10 5 / ml, drop into 96-well culture plate, 100μL per well, 37℃, 5%CO 2 cultured in an incubator.
[0051] 3. Cultivate overnight, and observe under a low-power microscope the next day. And spread subcloned cells 150ul.
[0052] (3) ...
Embodiment 2
[0066] Example 2 Sequencing of B7-H1 Antibody Heavy and Light Chain Variable Regions
[0067] 1. Extract the cDNA of hybridoma cells: extract RNA from the hybridoma cell line, and use RT-PCR technology to reverse-transcribe the obtained RNA into cDNA; use specially designed upstream and downstream primers to PCR clone the hybridoma cell chain variable region (mVH) and light chain variable region (mVL);
[0068] 2. mVH and mVL were respectively connected to the cloning vector (pJET cloning vector), and the connection product was transformed into competent bacteria DH5a. Since the pJET vector carries the ampicillin (Amp+) resistance gene, the transformed bacteria can be applied to the Amp-resistant LB solid culture medium, 37 ° overnight culture;
[0069] 3. The bacteria to be plated grow scattered colonies, select colonies with clear edges and good growth, and further sequence and identify them;
[0070] 4. According to the sequencing results, the candidate heavy and light ch...
Embodiment 3
[0079] Example 3 Karyotype Analysis of Hybridoma Cells
[0080]Take well-grown cells and add colchicine to make the final concentration 0.04~0.08μl / ml. After culturing for 2 hours, collect the cells (1000rpm, 10min) in a centrifuge tube, add 0.5ml of 37℃ pre-warmed 0.075mol / L KCl solution drop by drop, then add 5~10ml, blow gently with a pipette evenly, 37℃ Incubate for 20min. Add 1ml of freshly prepared fixative solution (3 parts of methanol plus 1 part of glacial acetic acid, prepared before use) to the tube, centrifuge at 1000rpm for 10min and discard the supernatant. Then add 8~10ml of fixative, pipette evenly, fix for 15~20min, centrifuge, and discard the supernatant. Then add 5ml of fixative and fix for 30min. Discard the supernatant by centrifugation, then add 1.5ml fixative, and pipette evenly. Take a glass slide frozen at -10°C, add 1-2 drops of cell suspension, and stain with fresh Giemsa solution (1 part of Giemsa stock solution plus 9 parts of 0.075mol / L, pH6.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com