Mixed electrolyte aqueous rechargeable nickel sodium/lithium battery and preparation method thereof
An electrolyte and lithium battery technology, applied in the field of electrochemistry, can solve the problem of high price of nickel-hydrogen batteries, and achieve the effects of being beneficial to mass production, reducing production costs and being easy to obtain.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
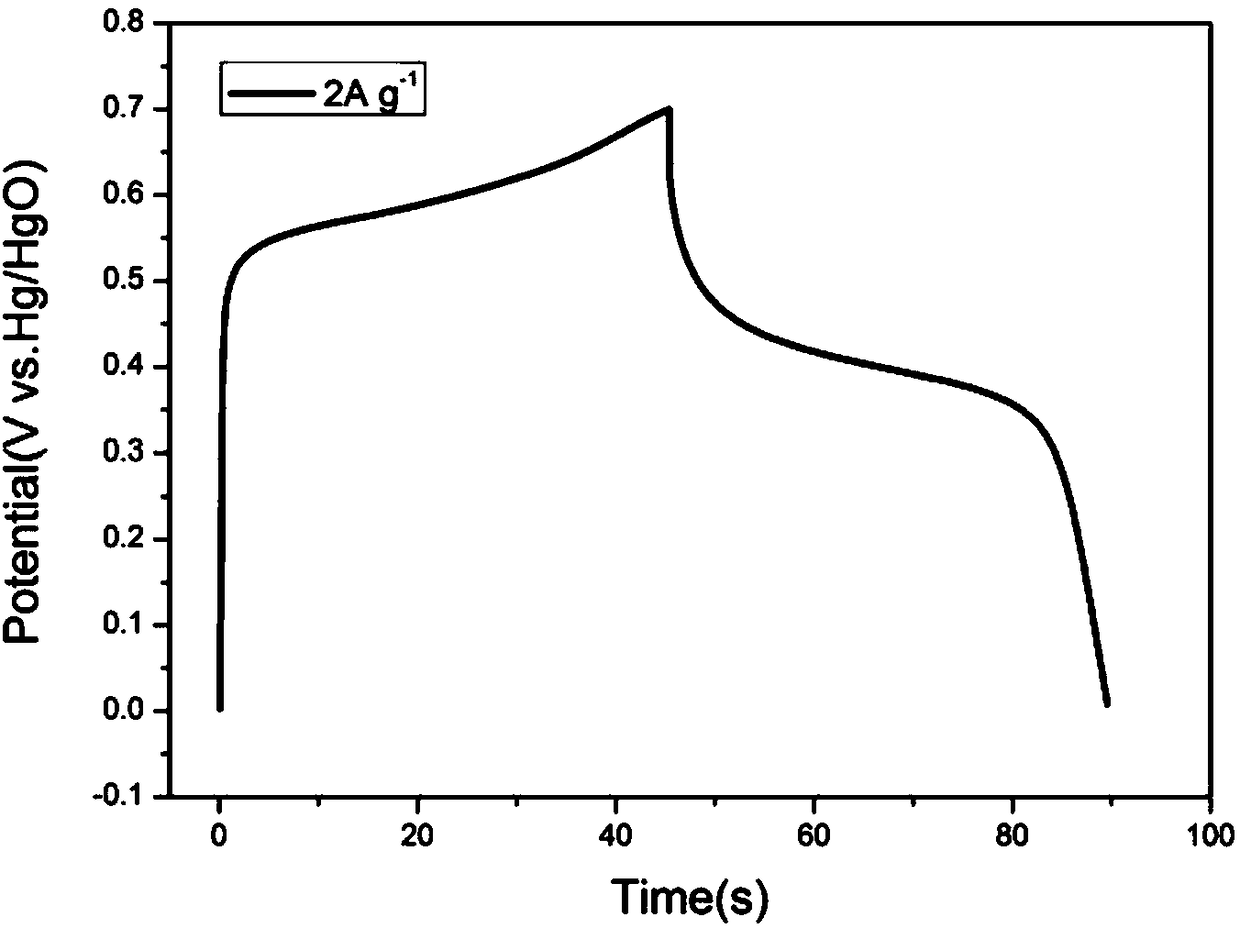
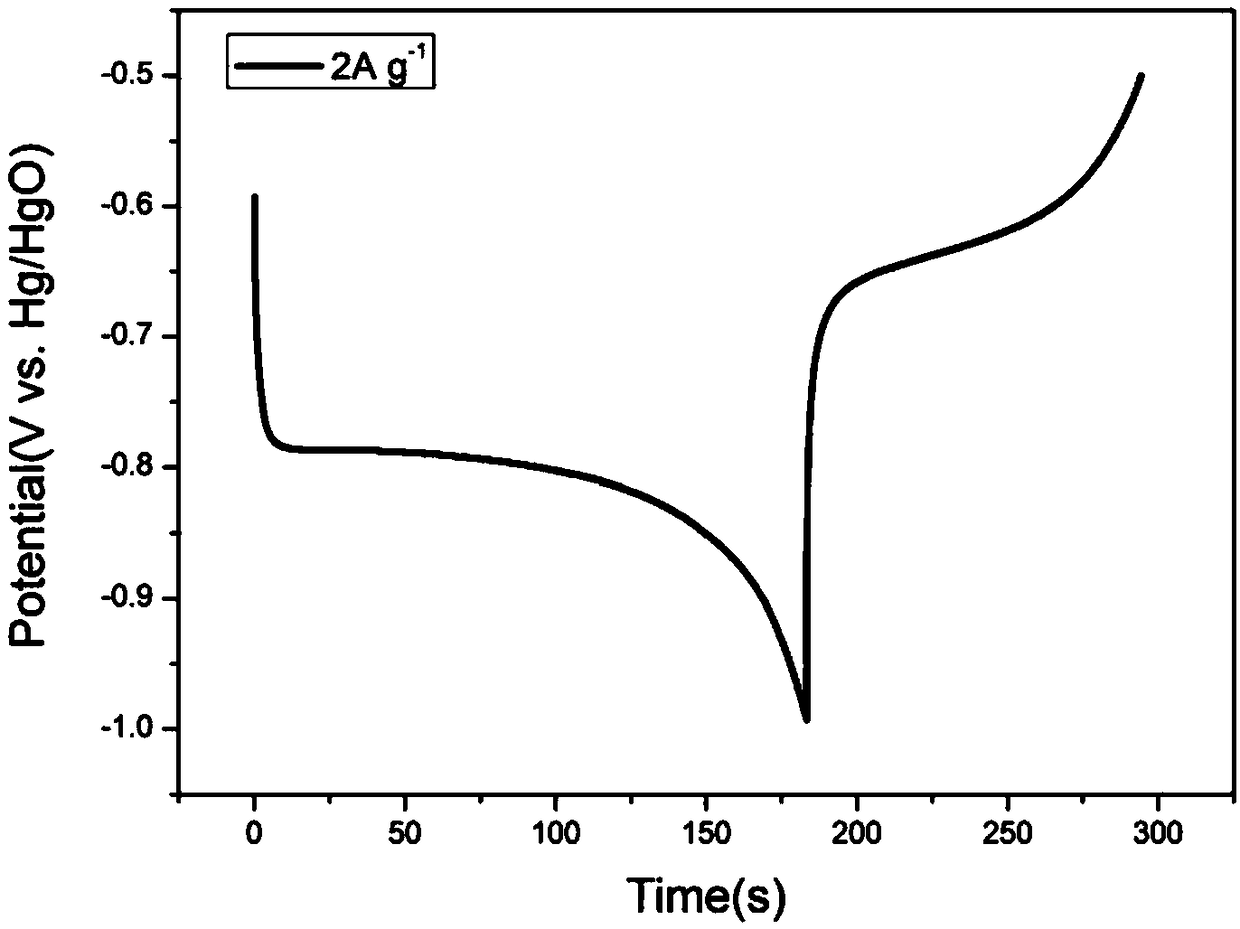
[0025] Such as Figure 1 to Figure 3 As shown, the present invention provides a kind of preparation method of mixed electrolyte water system rechargeable nickel-sodium / lithium battery, the electrolyte solution of water system neutral system and water system alkaline system battery is mixed, adopt Ni(OH) 2 As the cathode material, NaTi 2 (PO 4 ) 3 Is negative electrode material; Described preparation method comprises the steps:
[0026] Step 1, preparation of positive electrode material: mix 30ml 1.6mol / L NaOH with 30ml 0.8mol / L NiSO 4 ·6H 2 O mixed evenly and stirred for 1 hour, then transferred to a stainless steel reaction kettle and kept at 150°C-200°C for 12-24h, the obtained solution was centrifuged and cleaned, then freeze-dried to obtain the positive electrode material Ni(OH) 2 .
[0027] Step 2, preparation of negative electrode material: 3.4mL tetrabutyl titanate (C 16 h 36 o 4 Ti) was added dropwise to the solution containing 30-40mL ethylene glycol (C 2 h ...
Embodiment 1
[0032] Ni(OH) 2 -NaTi 2 (PO 4 ) 3 / C system
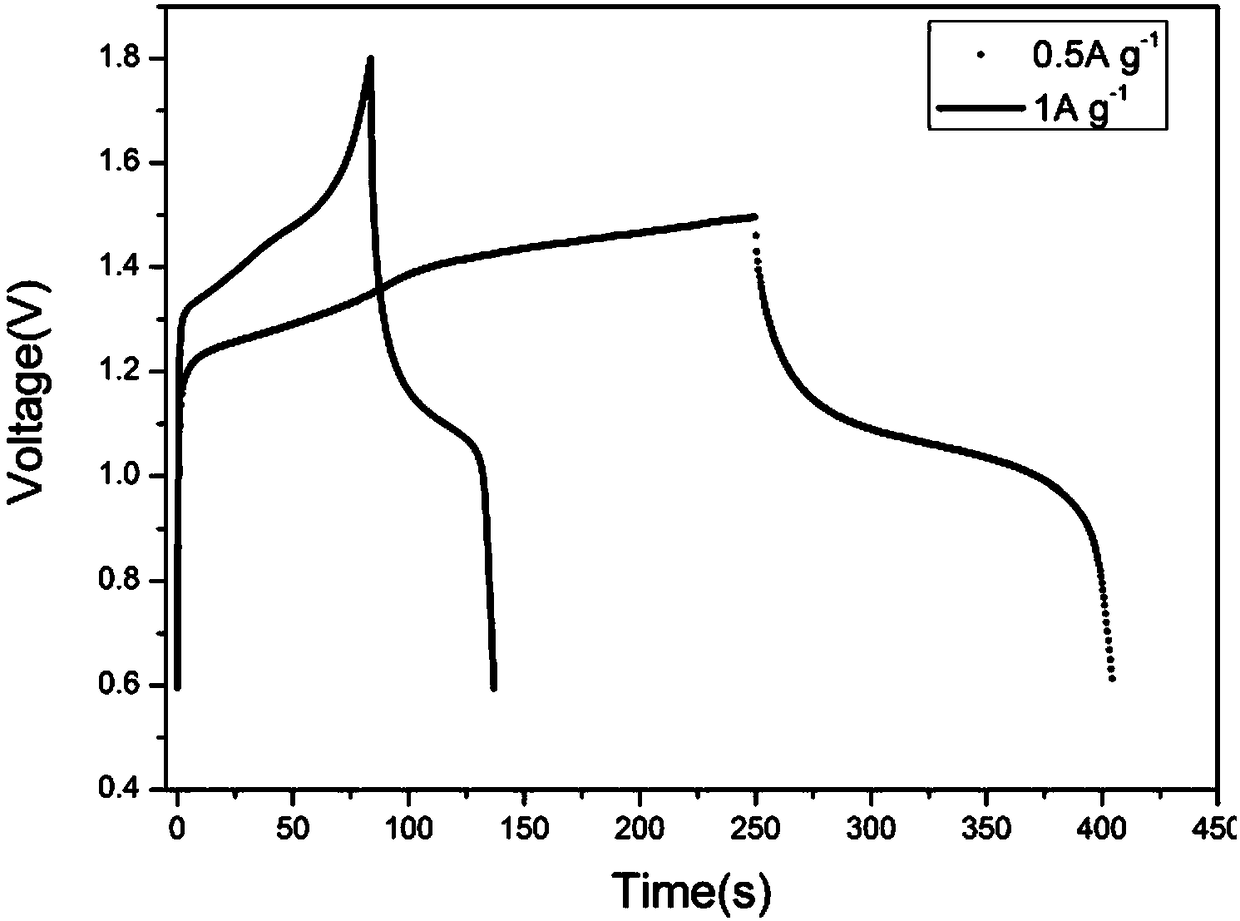
[0033] The positive electrode utilizes synthesized Ni(OH) 2 , the negative electrode uses NaTi 2 (PO 4 ) 3 / C, according to the mass ratio of active material: acetylene black: binder = 8:1:1, mix evenly, coat on the current collector (the positive electrode current collector is titanium mesh, the negative electrode current collector is stainless steel mesh), after drying , were pressed into the positive electrode film and the negative electrode film. Using non-woven fabric as the diaphragm, NaOH and Na 2 SO 4 The mixed aqueous solution is used as an electrolyte solution, and a full battery is assembled. The results show that its charging and discharging voltage is stable at about 1.40V and 1.10V, and at 0.5Ag -1 The charge capacity at the current density is 35mAh g -1 ,, discharge capacity is 24mAh g -1 . at 1Ag -1 Under the current density, the charging specific capacity is 25mAh g -1 , the specific discharge capac...
Embodiment 2
[0035] Ni(OH) 2 -NaTi 2 (PO 4 ) 3 / C system
[0036] The positive electrode utilizes commercial spherical Ni(OH) 2 , the negative electrode uses NaTi 2 (PO 4 ) 3 / C, according to the mass ratio of active material: acetylene black: binder = 8:1:1, mix evenly, coat on the current collector (the positive electrode current collector is titanium mesh, the negative electrode current collector is stainless steel mesh), after drying , were pressed into the positive electrode film and the negative electrode film. Using non-woven fabric as the diaphragm, NaOH and Na 2 SO 4 The mixed aqueous solution is used as an electrolyte solution, and a full battery is assembled. The results show that its charging and discharging voltage is stable at about 1.40V and 1.10V, and at 1Ag -1 Under the current density, the charge specific capacity is 23mAh g -1 , the specific discharge capacity is 17mAh g -1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Charging capacity | aaaaa | aaaaa |
| Discharge capacity | aaaaa | aaaaa |
| Charge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com