A carboxyl-containing organic photoelectric material and its preparation method and application
An organic photoelectric material, carboxyl technology, applied in the direction of photovoltaic power generation, circuits, electrical components, etc., can solve the problems of uneven conduction, uneven microscopic conduction of thin films, and reduced charge mobility, so as to improve charge mobility, reduce content, Improve the effect of hydrogen bonding interactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Dissolve PTB7 (20 mg) (Shenzhen Ruixun Photoelectric Material Technology Co., Ltd.) in 6 mL of toluene and 3 mL of 1,4-dioxane mixed solvent, slowly add 1 mL of saturated sodium hydroxide aqueous solution dropwise under stirring, and at 100 ° C After reacting for 5 h, the reaction solution was cooled to room temperature, and the reaction product was precipitated in dilute hydrochloric acid solution, and filtered to obtain black solid particles (15 mg).
[0028] (PTB7, where R 3 and R 4 For branched chain octyl);
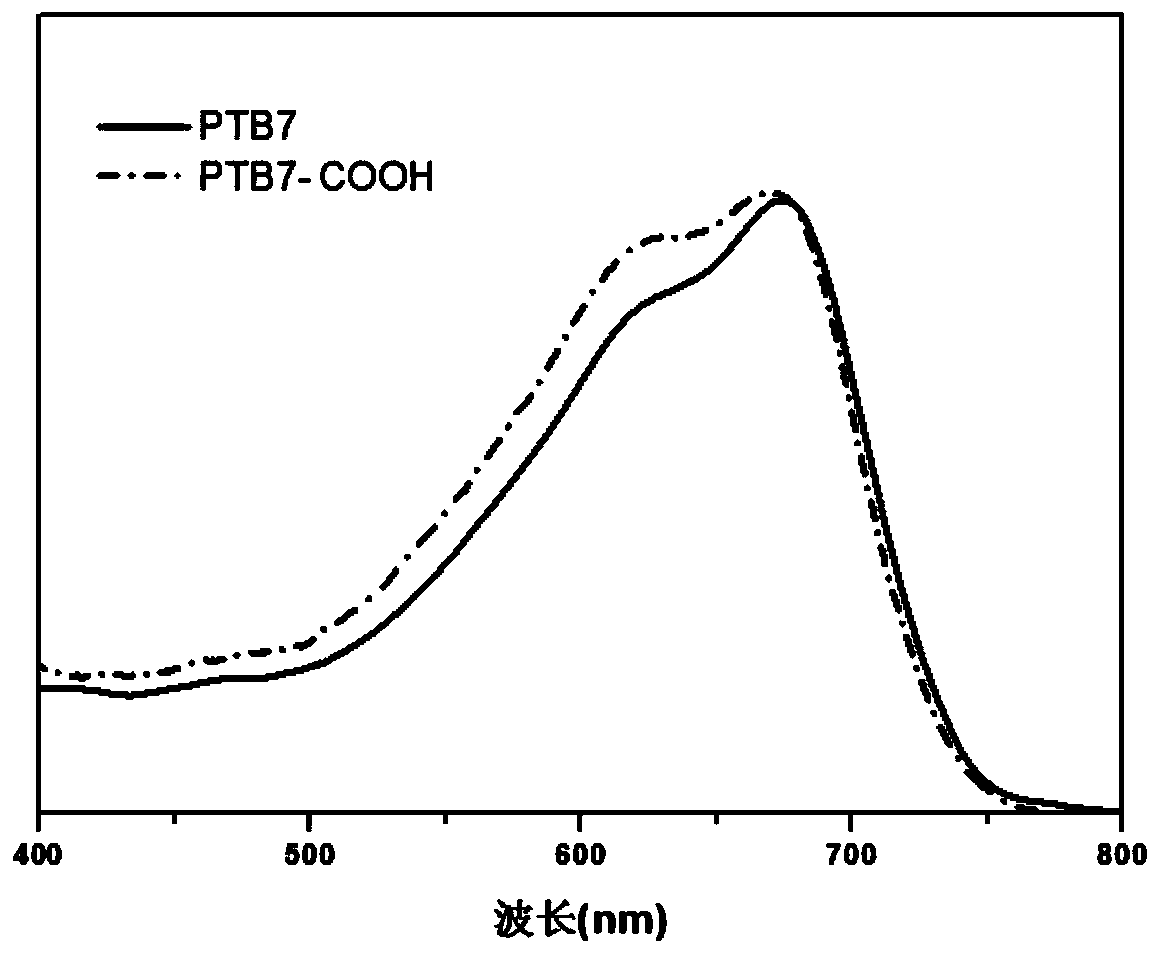
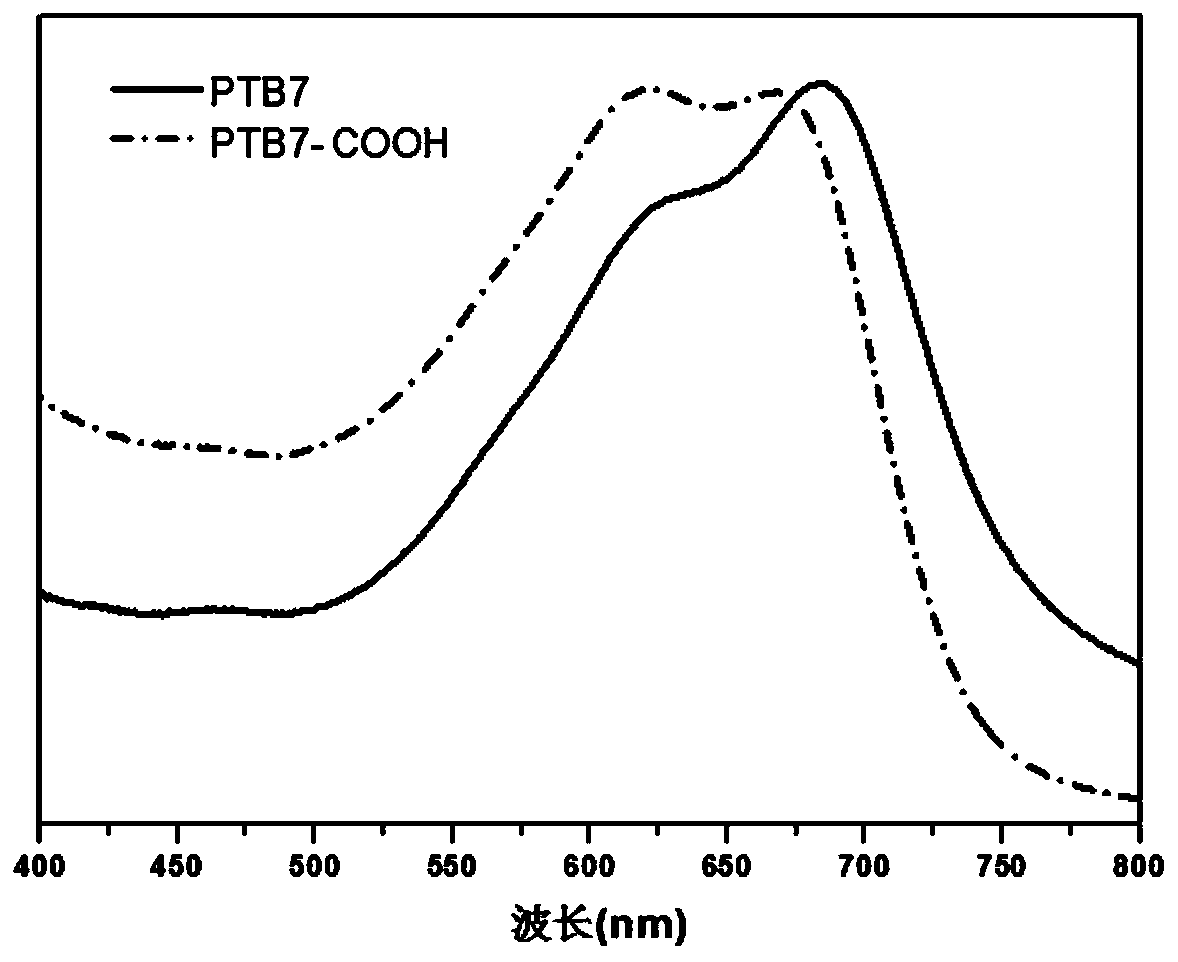
[0029] The solution (chlorobenzene solution) ultraviolet absorption spectrum of the product obtained in this embodiment and the thin film (the chlorobenzene solution of the sample is prepared by spin-coating and spinning film on quartz glass, and the rotating speed is 1000 rpm) ultraviolet absorption spectrum is respectively as follows figure 1 and figure 2 shown. Depend on figure 1 and figure 2 The results showed that under the action of alkali and ...
Embodiment 2
[0033] Dissolve PTB7-Th (50mg) (Shenzhen Ruixun Photoelectric Material Technology Co., Ltd.) in 12mL of toluene and 6mL of 1,4-dioxane mixed solvent, slowly add 3mL of saturated aqueous potassium hydroxide solution dropwise under stirring, at 90°C The reaction was carried out at high temperature for 10 h. After the reaction solution was cooled to room temperature, the reaction product was precipitated in dilute hydrochloric acid solution and filtered to obtain black solid particles (40 mg).
[0034] (PTB7-Th, where R 1 and R 2 For branched chain octyl);
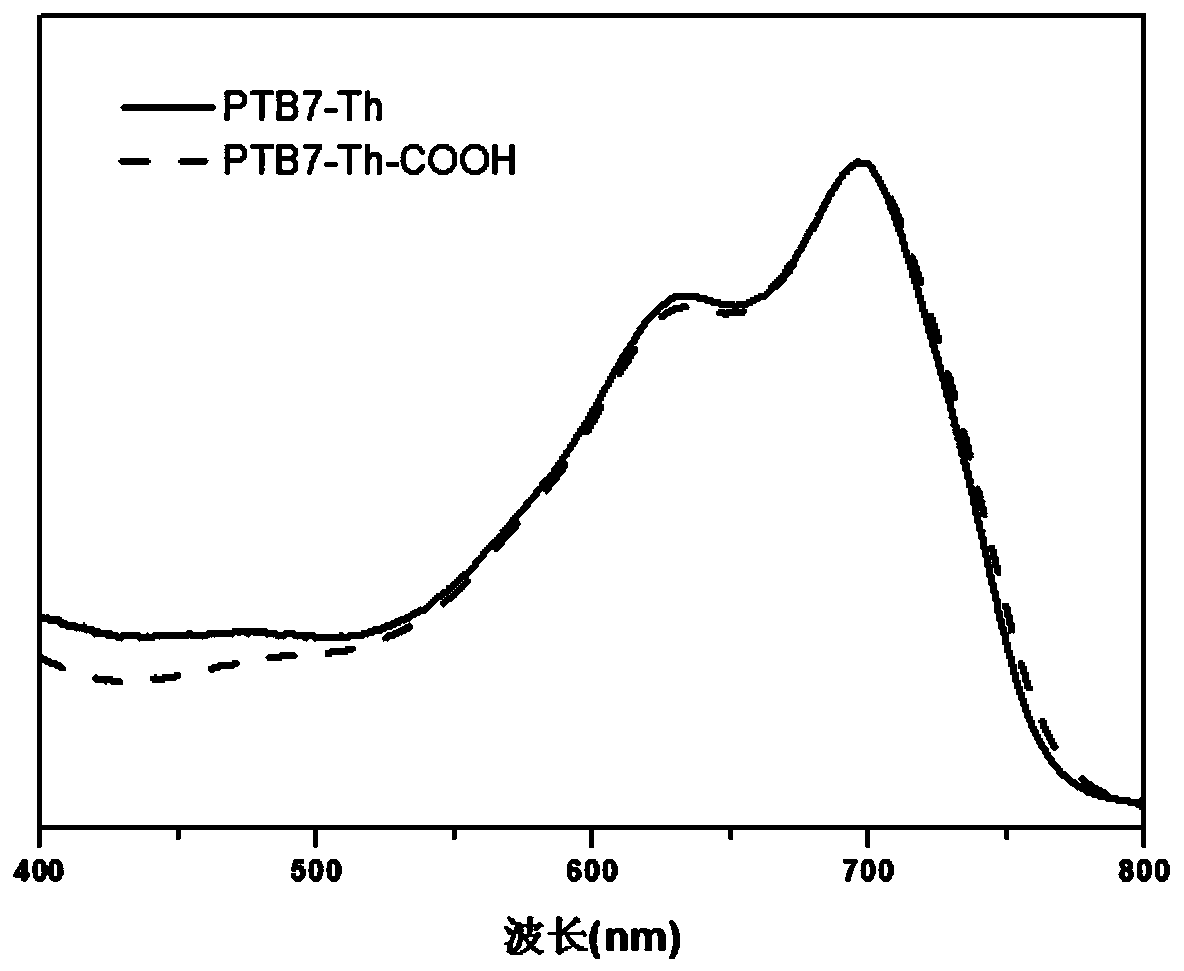
[0035] The solution (chlorobenzene solution) ultraviolet absorption spectrum of the product obtained in this embodiment and the thin film (the chlorobenzene solution of the sample is prepared by spin-coating and spinning film on quartz glass, and the rotating speed is 1000 rpm) ultraviolet absorption spectrum is respectively as follows image 3 and Figure 4 shown. Depend on image 3 and Figure 4 The results showed t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hole mobility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com