RAA constant temperature fluorescence detection method and reagent for infectious myonecrosis virus (IMNV)
A technique for muscle necrosis, detection kit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
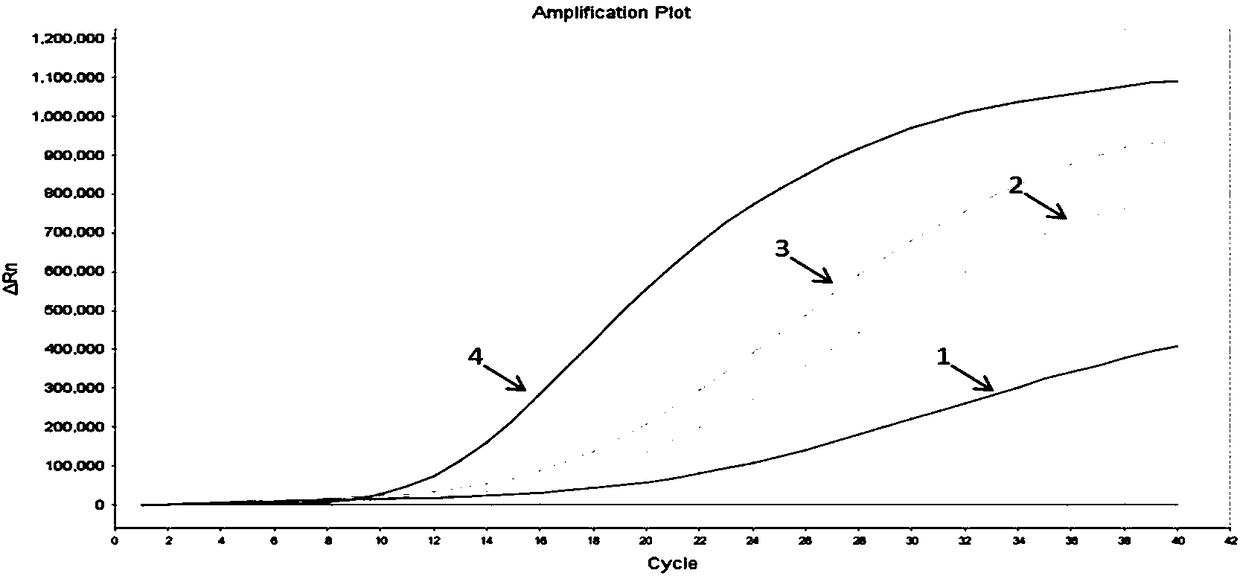
[0031]In the present invention, the gene sequence of the shrimp infectious myonecrosis virus strain is searched in the Genebank database, and DNAMAN 6.0 software is used to compare multiple sequences to find out the conserved segment. Four sets of primers and probes were designed in the conserved regions, and BLAST comparison was performed in the NCBI database. The sequences of the primers and probes are shown in Table 1. The positive sample amplification curve is as follows figure 1 shown.
[0032] Table 1 primers and probe sequences:
[0033]
[0034] Depend on figure 1 The results show that the amplification curves of the fourth group of primers and probes are the most typical, with obvious exponential phase and plateau phase, higher fluorescence intensity (ordinate value), and smaller CT value (the intersection of the curve and the threshold line Corresponding abscissa) result analysis is shown in Table 2. For other primers and probes, the rising height of the curve...
example 3
[0047] Example 3: Kit prawn infectious myonecrosis virus according to the present invention
[0048] 1. Extraction of positive sample nucleic acid
[0049] 1.1. Nucleic acid extraction: use traditional Trizol-RNA reagent or an equivalent RNA extraction kit.
[0050] 2. The configuration of the RAA reaction system: each test sample corresponds to a RAA reaction dry powder tube, and the reaction components and the added volume in each RAA reaction dry powder tube are shown in Table 3.
[0051] table 3:
[0052] RAA reaction system components
Volume (μL)
A Buffer
12.5μL
B Buffer
2.5μL
primer mix
4μL
specific fluorescent probe
0.6μL
DNA template
2μL
DEPC treated water
28.4μL
total capacity
50μL
[0053] A Buffer is 20% PEG; B Buffer is 280mM MgAc
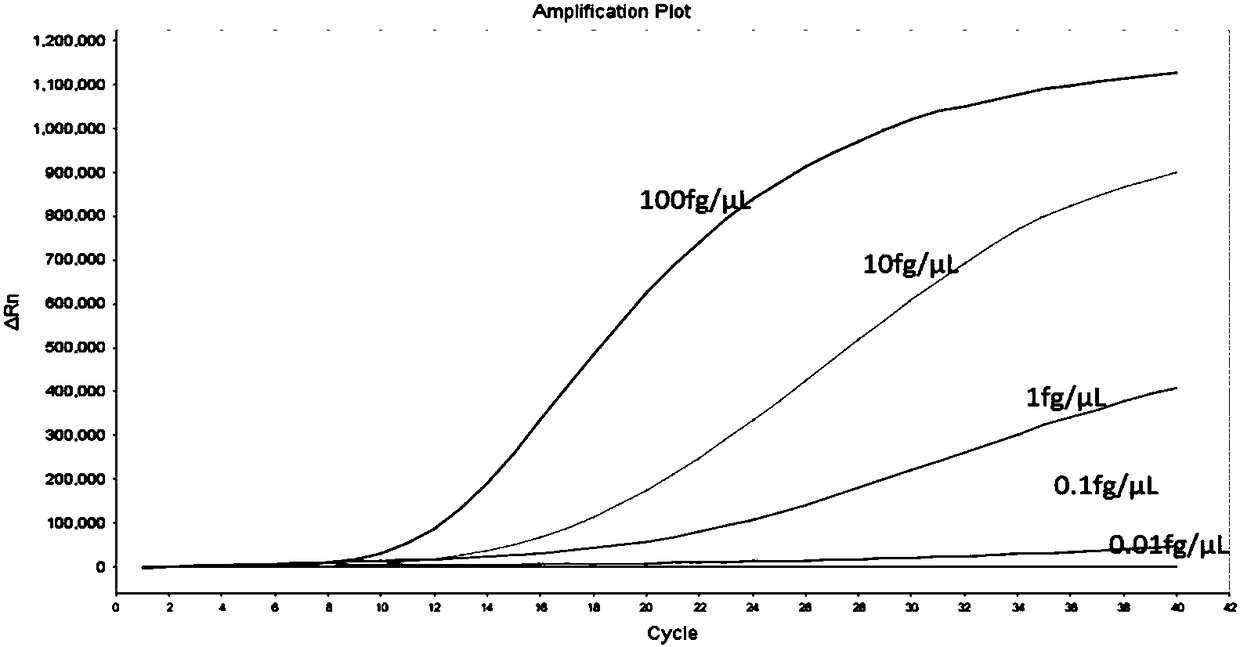
[0054] 3. Place the RAA reaction tube with the reaction system in the ABI7500 amplification instrument, and carry out RAA amplification ac...
Embodiment 4
[0057] Embodiment 4: Evaluation of the RAA detection kit of the present invention in clinical practice
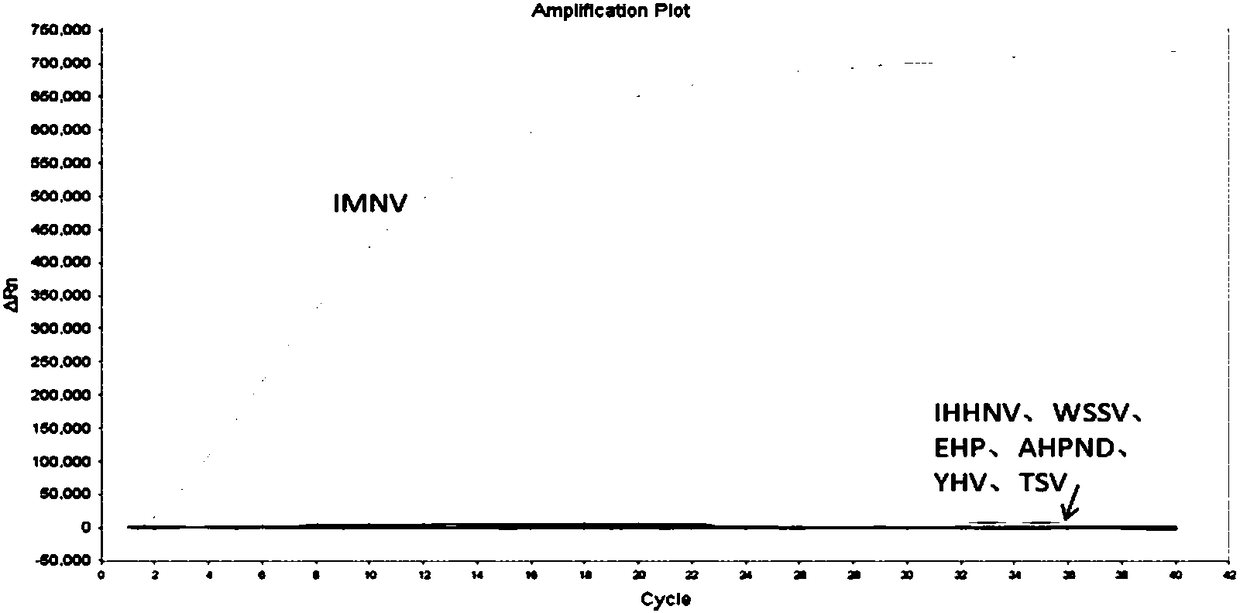
[0058] The kit of the present invention is used to carry out a clinical blind sample experiment, and 50 prawns are detected; the experimental results show that the fourth primer pair of the present invention can distinguish Hepatocystis prawns, and the positive coincidence rate with reverse transcription PCR is very high. Among the 50 samples, reverse transcription PCR, 38 samples were positive results, 12 samples were negative results, 39 samples were positive results by RAA method, 11 samples were also negative results, and one positive result was different. This sample was amplified by reverse transcription PCR and sequenced, and the sequencing result showed that the sample was positive, indicating that the RAA detection reagent of the present invention has a higher accuracy rate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com