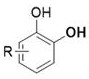
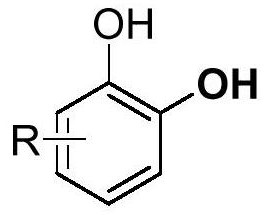
A class of catechol derivatives and preparation method thereof
A technology of catechol and derivatives, which is applied in the field of catechol derivatives and its preparation, can solve the problems that are difficult to achieve mass production, difficult to realize, etc., and achieve wide range of substrates, convenient operation, bottom The effect of variety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1, the preparation method of synthesizing 2-((5-nitropyrimidin-2-yl) oxygen group) phenyl acetate
[0034] The first step: vacuumize a dry 50ml reaction eggplant bottle with nitrogen three times, then add phenol (0.0941mg, 1.0mmol, 1.0equiv) to the reaction eggplant bottle, add 3.0ml of dried THF and stir until the phenol is completely Then add NaH (28.8mg, 1.2mmol, 1.2equiv, 60% suspension in mineral oil) to the reaction eggplant bottle under ice bath, and react for 30min under ice bath; then add 2-chloro- 5-Nitropyrimidine (0.1593 g, 1.0 mmol, 1.0 equiv). The whole mixture was slowly raised to 50°C for 12 hours. The process of the reaction was detected by TLC, if it was detected that all the phenol had reacted completely, the reaction could be stopped. The experimental treatment is to drain the solution in the reaction; dissolve the solute in the reaction eggplant bottle with ethyl acetate, and transfer it to a 100ml round bottom flask, and add 3ml (200-3...
Embodiment 2
[0046] Embodiment 2, the method for the preparation of synthetic 2-((5-nitropyrimidin-2-yl) oxy)phenyl acetate
[0047] The first step: vacuumize a dry 50ml reaction eggplant bottle with nitrogen three times, then add 4-tert-butylphenol (0.150g, 1.0mmol, 1.0equiv) to the reaction eggplant bottle, and add 3.0ml of dried Stir in THF until 4-tert-butylphenol is completely dissolved, then add NaH (28.8mg, 1.2mmol, 1.2equiv, 60% sodium hydride content suspended in mineral oil) to the reaction bottle under ice bath. React under ice bath for 30min; then add 2-chloro-5-nitropyrimidine (0.1593g, 1.0mmol, 1.0equiv) into the reaction flask. The whole mixture was slowly raised to 50°C for 12 hours. The process of the reaction was detected by TLC, if it was detected that all the phenol had reacted completely, the reaction could be stopped. The experimental treatment is to drain the solution in the reaction; dissolve the mixture in the reaction eggplant bottle with ethyl acetate, and tran...
Embodiment 3
[0052] Embodiment 3, the method for the preparation of synthetic 3-(tert-butyl)-2-((5-nitropyrimidin-2-yl)oxy)phenyl acetate
[0053] Step 1: Vacuum a dry 50ml reaction flask with nitrogen three times, then add 2-tert-butylphenol (150mg, 1.0mmol, 1.0equiv) to the reaction flask, add 3.0ml of dried THF Stir until 2-tert-butylphenol is completely dissolved, then add NaH (28.8mg, 1.2mmol, 1.2equiv, 60% sodium hydride content suspended in mineral oil) to the reaction bottle under ice bath, and store in ice bath React for 30 min; then add 2-chloro-5-nitropyrimidine (0.1593 g, 1.0 mmol, 1.0 equiv) into the reaction bottle. The whole mixture was slowly raised to 50°C for 12 hours. The process of the reaction was detected by TLC, if it was detected that all the phenol had reacted completely, the reaction could be stopped. The experimental treatment is to drain the solution in the reaction; dissolve the solute in the reaction eggplant bottle with ethyl acetate, and transfer it to a 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com