Method for preparing antigen substitute by using human fibronectin type III domain to display two antigenic epitopes
A fibronectin and domain technology, which is applied in the field of preparation of antigen substitutes based on the display of double antigenic epitopes of human fibronectin type III domain, can solve the problem of difficulty in antigen preparation, and achieve efficient preparation, short cycle and easy operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
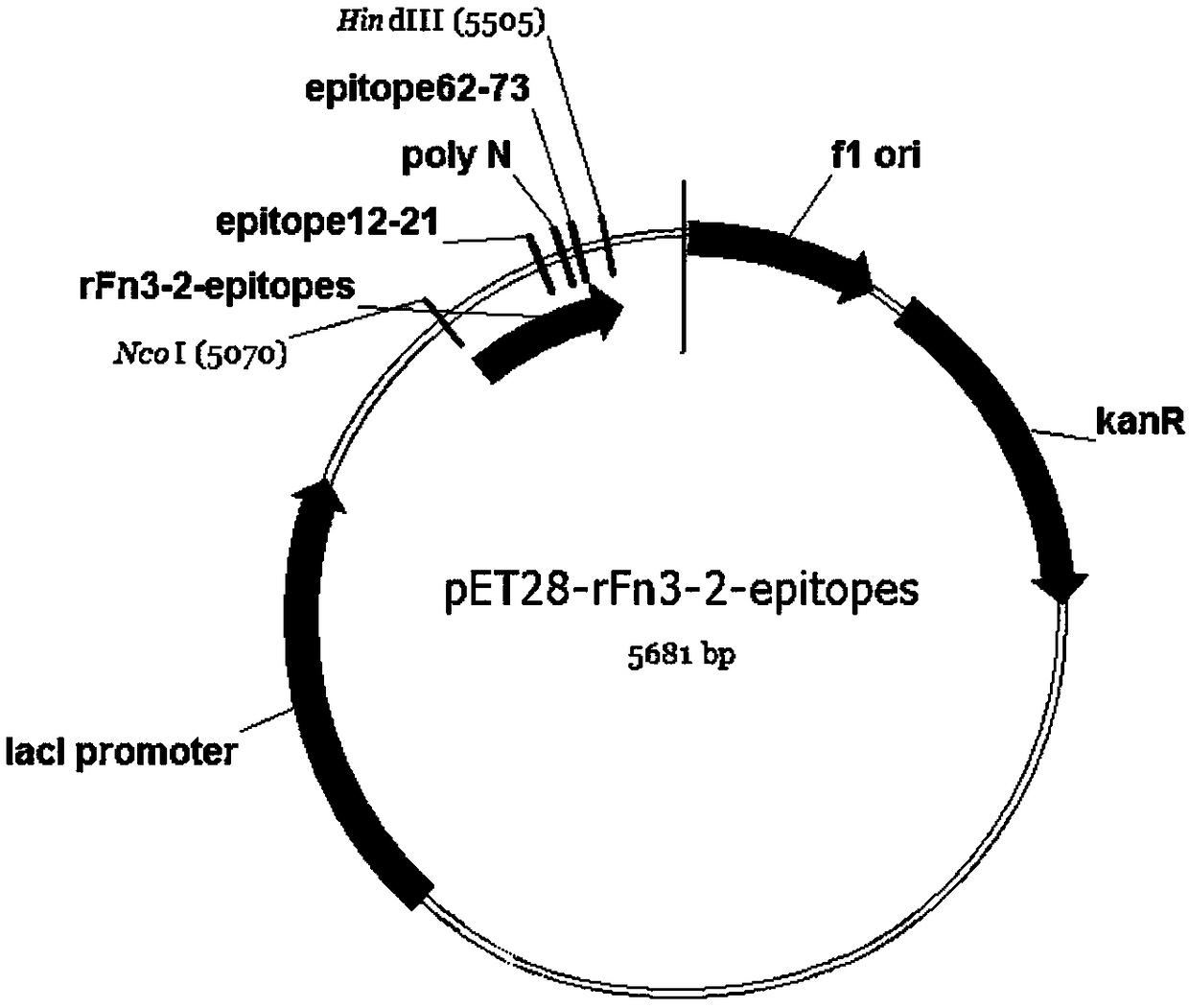
[0030] The construction of recombinant protein expression vector displaying NT-proBNP double epitope of human fibronectin type Ⅲ domain:
[0031] Replace the base sequence encoding the Fn3FG loop region with the base sequence encoding the NT-proBNP12-21 amino acid residue epitope (ie, the base sequence encoding Arg-Gly-Asp-Ser-Pro-Ala-Ser-Ser-Lys) , replacing the base sequence encoding the 3' end of Fn3 with the base sequence encoding the epitope of amino acid residues 62-73 of NT-proBNP, so as to realize the stable expression of the NT-proBNP dual epitope gene through the human fibronectin type III domain method. Introduce the base sequence encoding SSNNNNNNNNNNN (S represents serine residue, N represents asparagine residue) short peptide base sequence in encoding two short peptide base sequences, to prolong the distance between two antigenic epitopes, reduce two The interaction between them; the base sequence encoding 6 groups of amino acid residues is introduced at the 5' ...
Embodiment 2
[0034] Escherichia coli BL21(DE3) transformation:
[0035] 0.1 μg of the recombined plasmid pET28-rFn3-2-epitopes was heat-shocked to transform 100 μL of E. coli BL21 (DE3) chemically competent cells. The specific operation is: after gently mixing the above plasmids and chemically competent cells, ice bath for 3 minutes, then heat shock at 42°C for 80 seconds, ice bath for 5 minutes, and then add 200 μL of SOC medium preheated at 37°C; 37 After standing in the incubator for 30 minutes, culture at 37°C and 220rpm for 1 hour with shaking; take 50 μL and 100 μL of cultured bacterial liquid to gradient plate respectively, and the culture medium used is the antibiotic containing kanamycin (final concentration: 50 μg / mL). LB solid medium. Incubate overnight in a 37°C incubator to grow positive single clones.
[0036] Among them, the formula of LB solid medium is: tryptone (Tryptone) 10g / L, yeast extract (Yeasextract) 5g / L, sodium chloride (NaCl) 10g / L, agarose powder 15g / L, and dd...
Embodiment 3
[0038] Induced expression of human fibronectin type III domain displaying NT-proBNP dual epitope recombinant protein:
[0039] The positive transformants obtained in Example 2 were picked and streaked on LB solid medium containing kanamycin antibiotic (final concentration: 50 μg / mL), and cultured overnight in an incubator at 37°C. Single clones were picked and inoculated in 3 mL LB liquid medium containing kanamycin antibiotic (final concentration: 50 μg / mL), and cultured overnight at 37° C. and 220 rpm. The next day, inoculate the bacterial solution into 500 mL LB liquid medium containing kanamycin antibiotic (final concentration 50 μg / mL) at a ratio of 1:100, and cultivate to OD at 37 ° C and 220 rpm 600nm reach 0.5. Then, isopropylthiogalactopyranoside (IPTG) was added with a final concentration of 0.2 mM, and the expression was induced at 26° C. and 220 rpm for 4 hours. During this process, the recombinant protein rFn3-2-epitopes was expressed intracellularly in Escheric...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com