Human placenta mesenchymal stem cell suspension protection agent
A technology of stem cells and human placenta, applied in the preservation, application, and animal husbandry of human or animal bodies, can solve the problems of rapid decline in cell activity, lack of nutrition and buffer capacity, and easy aggregation of cells to achieve maintenance The effect of stem cell activity, large-scale production, and uniform cell shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
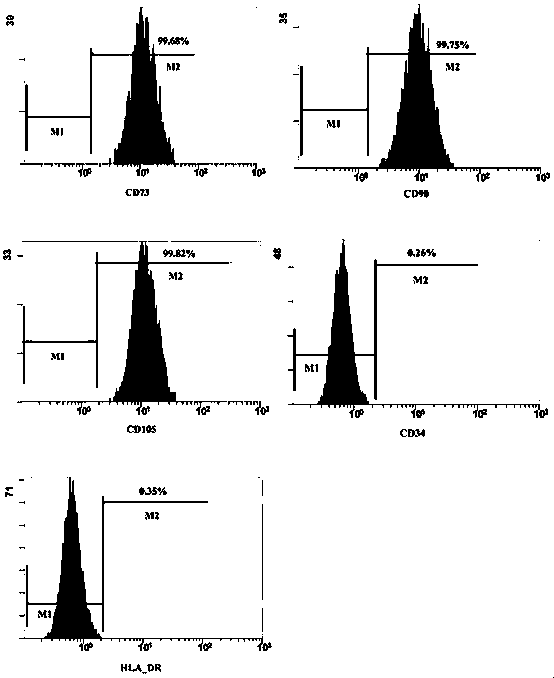
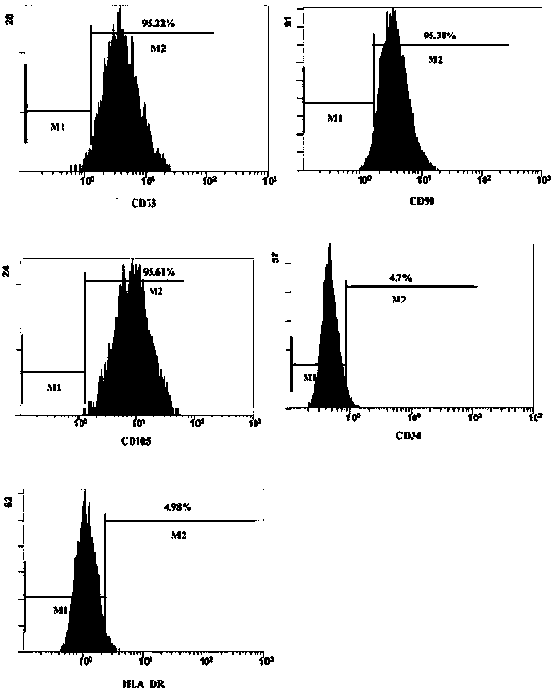
Image
Examples
preparation example Construction
[0020] (1) Preparation of the base liquid: Dilute and mix the dextran sodium chloride injection and the NaCl solution for injection at a ratio of 1:1 to complete the preparation of the base liquid. The concentration of the dextran sodium chloride injection is 6%. The concentration of NaCl solution is 0.9%;
[0021] (2) Addition of ingredients: add human serum albumin, trehalose and vitamin C to the above base liquid in sequence, the amount of human serum albumin is 2-10%, the amount of trehalose is 0.001-0.005%, vitamin The amount of C added is 0.01-0.05%, fully oscillated and mixed, the oscillating time is 30 s-1 min, and the oscillating speed is 1820 rpm, and the human placental mesenchymal stem cell suspension protective agent is prepared;
[0022] (3) Storage: Place the prepared human placental mesenchymal stem cell suspension protective agent in a 500 ml sterile medium bottle and store it at 4°C.
Embodiment 1
[0024] Preparation of placental stem cells
[0025] (1) Collection of human placenta tissue: the placenta was taken from a full-term healthy pregnant woman whose HBV antigen, anti-HCV antibody, anti-HIV antibody, anti-Treponema pallidum antibody, ALT, mycoplasma and other test items were all negative, and the placenta was removed from the operating table Afterwards, immersed in a special placenta preservation solution, maintained at 2-8°C for transportation, and separated within 24 hours;
[0026] (2) Tissue disinfection and washing: Take the placenta out of the preservation solution, place it flat on a sterile tray with the umbilical cord facing upward, cut off the umbilical cord, peel off the amniotic membrane, cut the chorionic tissue 0-0.3 cm thick under the amniotic membrane, and put Put it into a sterile petri dish, peel off the decidua basalis tissue, cut off the terminal villi clusters, and wash twice with 0.9% normal saline;
Embodiment 2
[0037] A kind of human placenta mesenchymal stem cell suspension protection agent
[0038]Take 6% low-molecular-weight dextran sodium chloride injection and 0.9% NaCl solution for injection, dilute and mix them 1:1, then use it as the base solution, add human serum albumin, trehalose, vitamin C, and human serum albumin into the base solution The volume ratio of trehalose is 2%, the mass percentage of trehalose is 0.001%, and the mass percentage of vitamin C is 0.01%. After fully mixing, the cell protection agent is prepared, and the prepared cell protection agent is placed in a 500 ml sterile medium bottle. Store at 4°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com