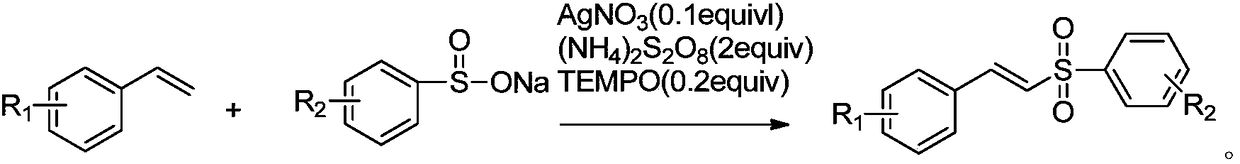
Method for synthesizing (E)-(2-(phenylsulfonyl)vinyl)benzene and derivative thereof
A technology of benzenesulfonyl and derivatives, applied in the field of synthesis-vinyl)benzene and derivatives thereof, can solve the problems of low yield, high cost, harsh reaction conditions, etc., and achieves cost reduction, yield improvement, and reaction conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Synthesis of (E)-(2-(phenylsulfonyl)vinyl)benzene
[0028] The substrate 31.2mg (0.3mmol) styrene, sodium benzene sulfinate 98.4mg (0.6mmol), the catalyst 5.1mg (0.03mmol) AgNO 3 , Oxidant 137.0mg (0.6mmol) (NH 4 ) 2 S 2 O 8 , TEMPO 5.4mg (0.06mmol). Add 1 mL of solvent toluene into a 25 mL sealed tube under air. Then put the sealed tube in an oil bath at 100°C for 2h. After the reaction, the reaction solution was cooled to room temperature. Add 40 mL of saturated brine, extract three times with 100 mL of ethyl acetate, and combine the organic layers. The organic layer was dried with anhydrous sodium sulfate, filtered, and the filtrate was distilled under reduced pressure and separated by silica gel column chromatography (ethyl acetate / petroleum ether: 1 / 8 as eluent) to obtain 51.3 mg of yellow liquid with a yield of 89%.
Embodiment 2
[0029] Example 2: Synthesis of (E)-1-chloro-4-(2-(benzenesulfonyl)vinyl)benzene
[0030] The substrate 41.6mg (0.3mmol) p-chlorostyrene, sodium benzenesulfinate 98.4mg (0.6mmol, catalyst 5.1mg (0.03mmol) AgNO 3 , Oxidant 137.0mg (0.6mmol) (NH 4 ) 2 S 2 O 8 TEMPO 5.4 mg (0.06 mmol). 1 mL of solvent toluene was added to a 25 mL sealed tube under air. Then put the sealed tube in an oil bath at 100°C for 2h. After the reaction, the reaction solution was cooled to room temperature. Add 40 mL of saturated brine, extract three times with 100 mL of ethyl acetate, and combine the organic layers. The organic layer was dried with anhydrous sodium sulfate, filtered, and the filtrate was distilled under reduced pressure and separated by silica gel column chromatography (ethyl acetate / petroleum ether: 1 / 8 as eluent) to obtain 63.5 mg, with a yield of 86%.
[0031] 1 H NMR (400MHz, CDCl3) δ 7.86 (d, J = 7.7 Hz, 2H), 7.56 (d, J = 15.4 Hz, 1H), 7.55 (d, J = 8.6 Hz, 1H), 7.49 (t, J =7.7Hz, 2H), 7...
Embodiment 3
[0034] Example 3: Synthesis of (E)-2-chloro-4-(2-(benzenesulfonyl)vinyl)benzene
[0035] The substrate 41.6mg (0.3mmol) o-chlorostyrene, sodium benzenesulfinate 98.4mg (0.6mmol), the catalyst 5.1mg (0.03mmol) AgNO 3 , Oxidant 137mg (0.6mmol) (NH 4 ) 2 S 2 O 8 , TEMPO 5.4mg (0.06moml). 1mL of solvent toluene was added to 25mL sealed tube under air. Then put the sealed tube in an oil bath at 100°C for 2h. After the reaction, the reaction liquid was cooled to room temperature. Add 40 mL of saturated brine, extract three times with 100 mL of ethyl acetate, and combine the organic layers. The organic layer was dried with anhydrous sodium sulfate, filtered, and the filtrate was distilled under reduced pressure and separated by silica gel column chromatography (ethyl acetate / petroleum ether: 1 / 8 as eluent) to obtain 67.7 mg, with a yield of 81%.
[0036] 1 H NMR(400MHz, CDCl 3 )δ8.02(d,J=15.4Hz,1H),7.90(d,J=8.0Hz,2H),7.57(t,J=7.3Hz,1H),7.50(t,J=7.7Hz,2H) ,7.44(d,J=7.7Hz,1H), 7.36(d,J=8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com