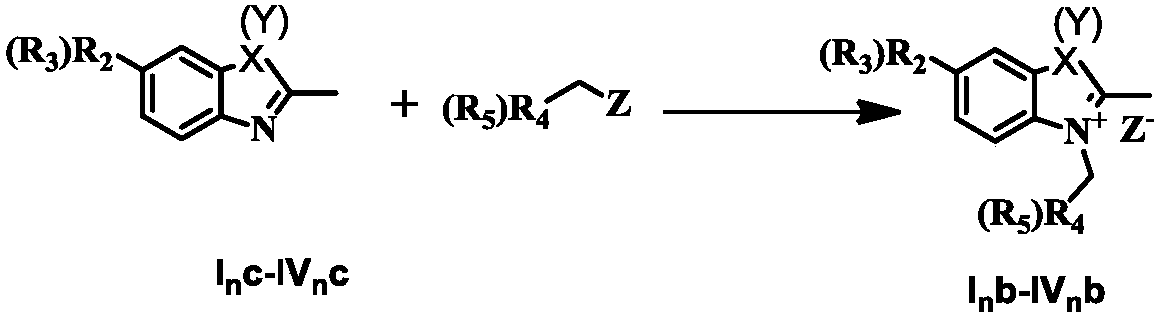Pentamethine cyanine dye and preparation method thereof
A technology of pentamethine and cyanine dyes, applied in the direction of methine/polymethine dyes, organic dyes, chemical instruments and methods, etc., can solve the problem of lack of super-resolution fluorescent materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
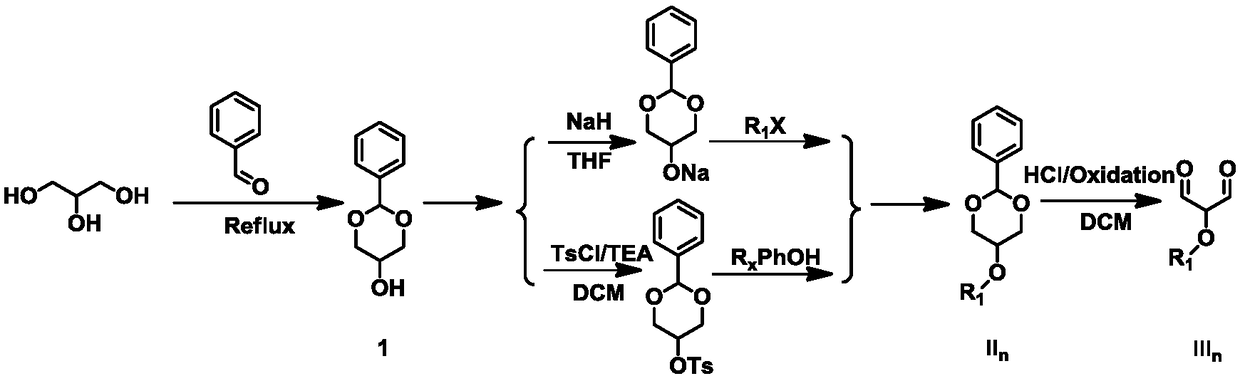
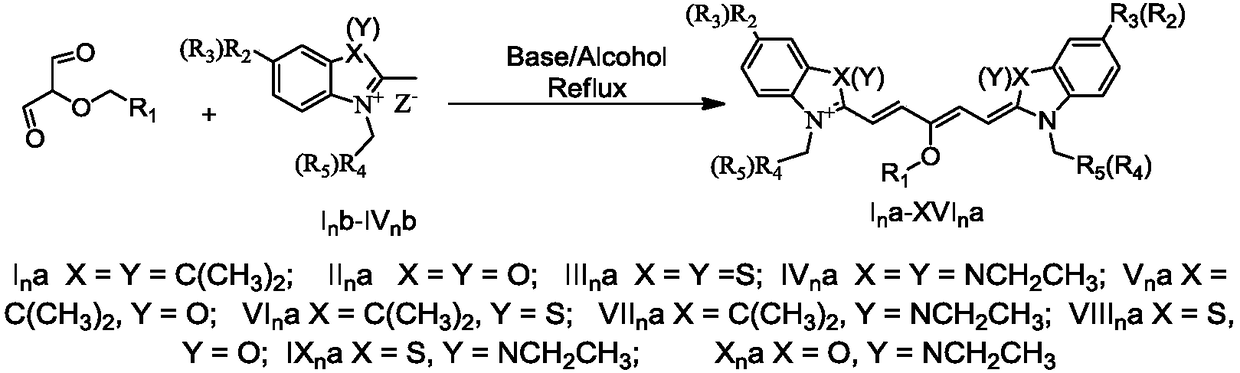
[0026] The present invention provides a pentamethyl cyanine dye and a preparation method. Due to the large molar light absorption of pentamethyl cyanine, the wavelength of absorption and emission can be adjusted, the synthesis is easy, the fluorescence quantum yield is low, and the characteristics of low cytotoxicity are applied as a fluorescent material. In addition, in addition to the above fluorescent properties, pentamethine dyes can also undergo photoinduced scintillation, which is suitable for super-resolution fluorescent materials required by STORM and SOFI.
[0027] Specifically, R 6 for (CH 2 ) n R 8 or CH 2 C 6 h 4 R 8 .
[0028] Specifically, R 7 is H, ethynyl, vinyl, phenyl, naphthyl, imidazole, pyrazole, oxazole, thiazole, furan, pyrrole heterocycle, C 6 h 4 R 9 , phenylboronic acid alcohol ester, propargyl, folic acid, methotrexate, RGD, Biotin, SO 3 R 10 or COOR 11 any of the.
[0029] Specifically, R 8 H, SO 3 R 10 or COOR 11 any of the.
[...
Embodiment 1
[0072] Synthesis of 2,3,3-trimethyl-3H-indoline quaternary ammonium salt (X=Y=C(CH 3 ) 2 )
[0073] Take 100g2,3,3-trimethyl-3H-indoline / 5-substituted-2,3,3-trimethyl-3H-indoline and dissolve it in 200g toluene, add 150g p-toluenesulfonate, in Under a nitrogen atmosphere, heat to reflux at 70°C for 6 h, cool to room temperature, add diethyl ether to wash, filter, and dry to obtain a pink solid (yield 75%).
Embodiment 2
[0075] Synthesis of 2,3,3-trimethyl-3H-indoline quaternary ammonium salt (X=Y=C(CH 3 ) 2 )
[0076] Dissolve 100g of 5-substituted-2,3,3-trimethyl-3H-indoline in 200g of o-dichlorobenzene, add 150g of sulfuric acid ester, heat and reflux at 90°C for 36h under a nitrogen atmosphere, cool to room temperature, and add ether Washed, filtered and dried to obtain a powdery white solid (yield 79%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com