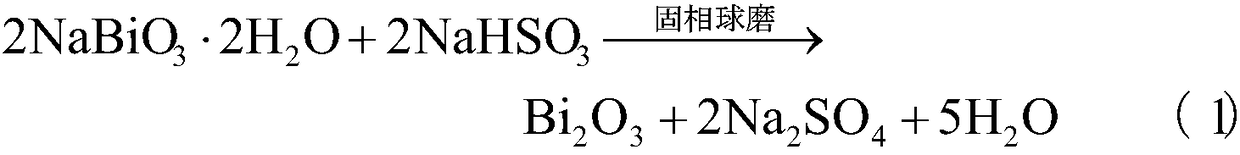Preparation method of bismuth (III) trioxide-bismuth oxycarbonate nano complex through room temperature solid phase chemical reactions
A technology of bismuth oxycarbonate nanometer and bismuth trioxide, which is applied in chemical instruments and methods, bismuth compounds, chemical/physical processes, etc., can solve the problems of reducing the active surface of products, hard agglomeration of product particles, and reducing product performance, etc. The effect of easy industrial production, simple preparation process and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Weigh 0.01 mole of sodium bismuthate dihydrate and 0.01 mole of sodium bisulfite according to a molar ratio of 1:1 and add them to a 50 mL zirconia ball mill jar equipped with 50 zirconia balls with a diameter of 6 mm and 8 zirconia balls with a diameter of 10 mm. Continuous ball milling in QM-3SP04 planetary high-energy ball mill at 480rpm for 10 hours, the product was washed with distilled water, centrifuged and vacuum-dried at 60°C and 0.1Mpa vacuum for 2 hours to obtain the target product.
Embodiment 2
[0022] Weigh 0.01 mole of sodium bismuthate dihydrate and 0.01 mole of sodium bisulfite at a molar ratio of 1:1 and add them to a 50 mL zirconia ball mill jar equipped with 50 zirconia balls with a diameter of 6 mm and 8 zirconia balls with a diameter of 10 mm. Continuous ball milling in QM-3SP04 planetary high-energy ball mill at 480rpm for 15 hours, the product was washed with distilled water, centrifuged and vacuum-dried at 60°C and 0.1Mpa vacuum for 2 hours to obtain the target product.
Embodiment 3
[0024] Weigh 0.01 mole of sodium bismuthate dihydrate and 0.01 mole of sodium bisulfite at a molar ratio of 1:1 and add them to a 50 mL zirconia ball mill jar equipped with 50 zirconia balls with a diameter of 6 mm and 8 zirconia balls with a diameter of 10 mm. Continuous ball milling in QM-3SP04 planetary high-energy ball mill at 480rpm for 20 hours, the product was washed with distilled water, centrifuged and vacuum-dried at 60°C and 0.1Mpa vacuum for 2 hours to obtain the target product.
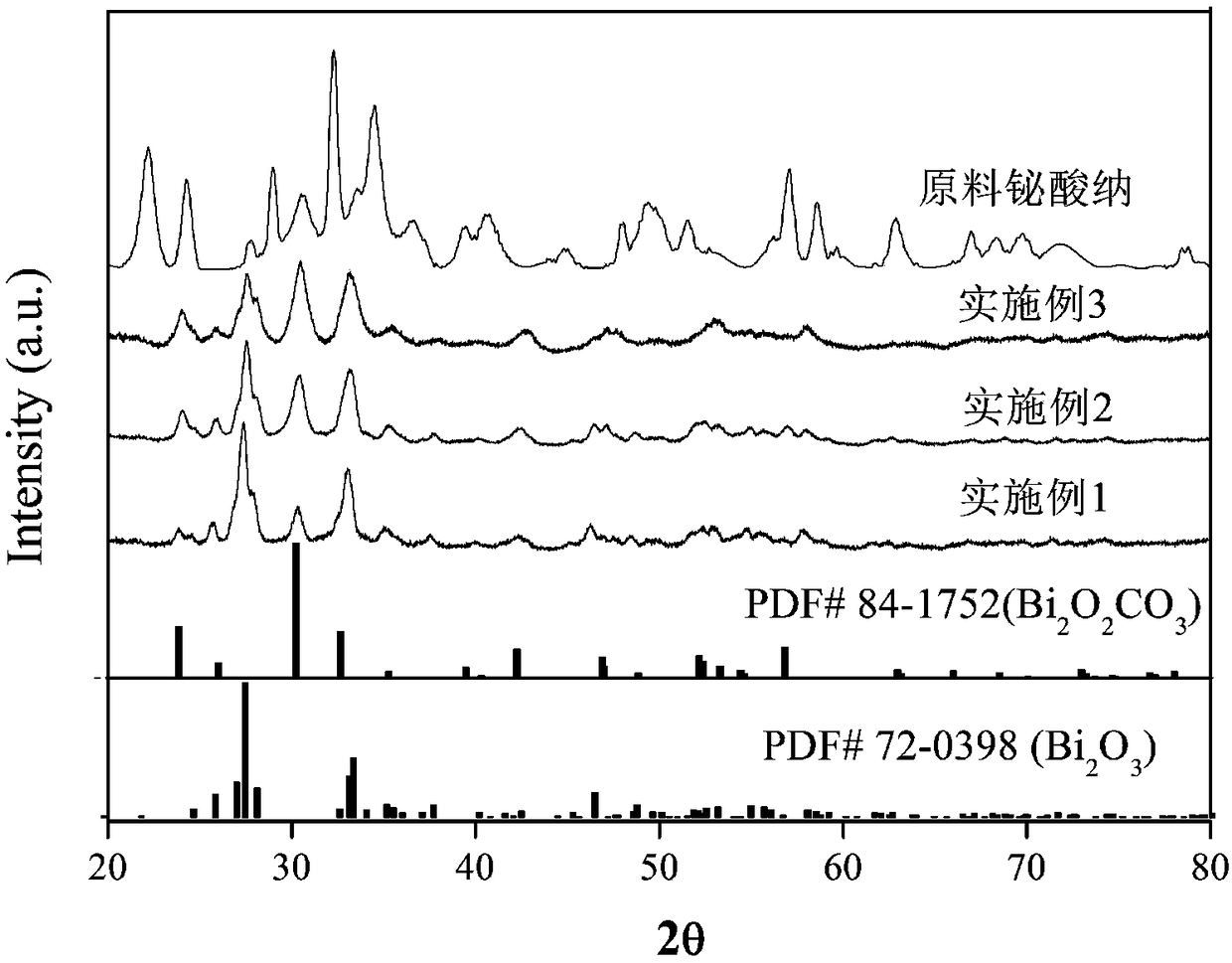
[0025] The X-ray diffraction analysis (XRD analysis) of above-mentioned embodiment gained target product: the target product that embodiment 1,2 and 3 are made and raw material sodium bismuthate dihydrate are carried out XRD analysis respectively, and the results are shown in figure 1 , it can be seen that only Bi 2 o 3 and Bi 2 o 2 CO 3 There is no characteristic diffraction peak of the raw material sodium bismuthate, indicating that the solid phase reaction between sodium bismuthate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com