Positive electrode material of lithium-sulfur battery, preparation method thereof and lithium-sulfur battery
A lithium-sulfur battery and cathode material technology, which is applied in battery electrodes, secondary batteries, circuits, etc., can solve problems such as poor conductivity of cathode materials, polysulfide shuttle effect, sulfur cathode volume effect, and reduction of cathode material volumetric energy density.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
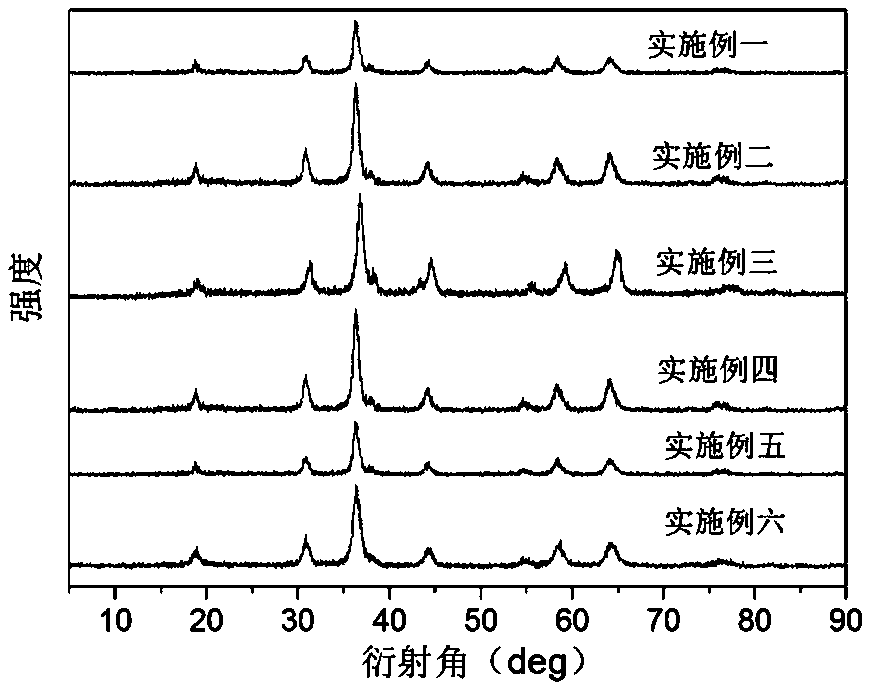
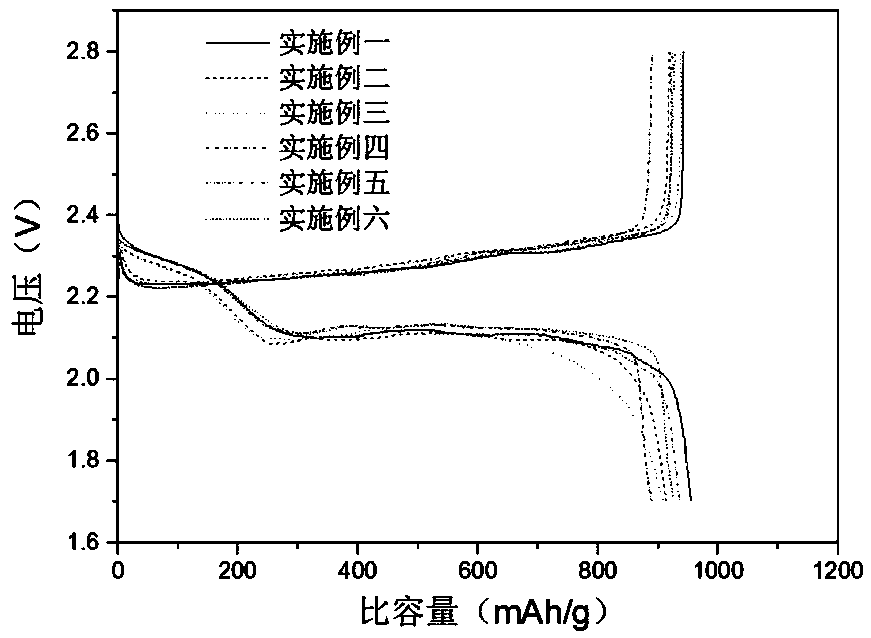
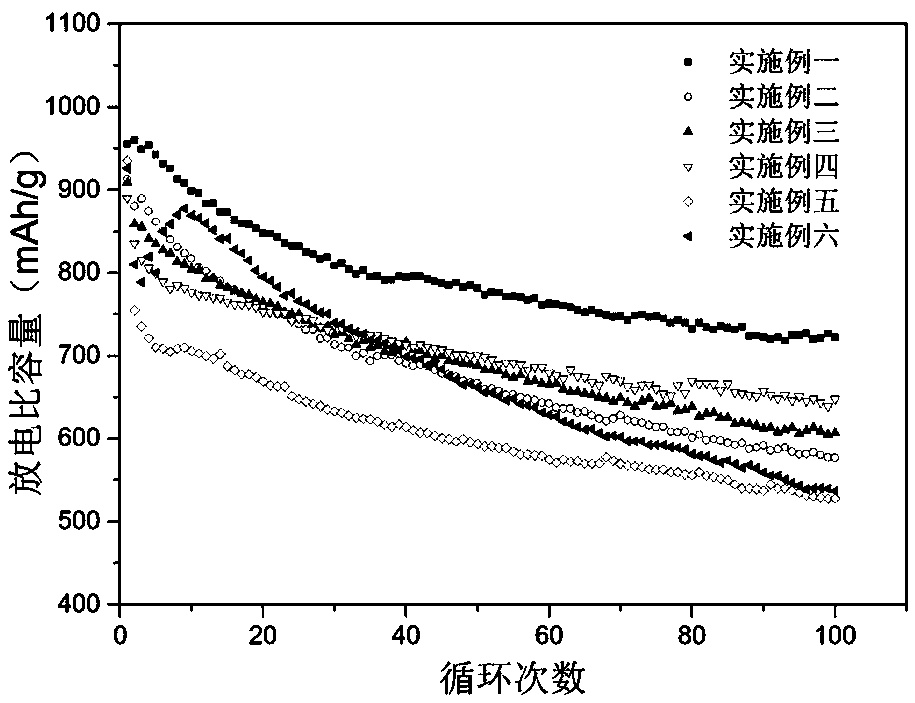
Examples
Embodiment 1
[0044]A cobaltate loaded sulfur-lithium-sulfur battery composite positive electrode material, specifically prepared according to the following steps:
[0045] Step 1, magnesium cobaltate (MgCo 2 o 4 ) preparation:
[0046] Measure 10 mL of deionized water and 40 mL of absolute ethanol and mix evenly.
[0047] Weigh 0.5 mmol of cobalt acetate and 0.25 mmol of magnesium acetate, add them to the above mixed solution, and continue stirring to dissolve.
[0048] Add 0.5 mL of 25% aqueous ammonia dropwise.
[0049] The mixed solution was transferred to a 100 mL hydrothermal reactor and placed in an oven at 200 °C for 15 h.
[0050] The hydrothermal reaction product was centrifugally washed with deionized water and ethanol three times each, and placed in a vacuum drying oven at a temperature of 60 °C with a vacuum degree of -0.1 MPa.
[0051] The dried material was placed in a muffle furnace at 500 °C for 2 h to obtain magnesium cobaltate.
[0052] Step 2, the magnesium cobalta...
Embodiment 2
[0057] A copper cobaltate loaded sulfur-lithium-sulfur battery composite positive electrode material, specifically prepared according to the following steps:
[0058] Step 1, copper cobaltate (CuCo 2 o 4 ) preparation:
[0059] Measure 10 mL of deionized water and 40 mL of absolute ethanol and mix evenly.
[0060] Weigh 0.5 mmol of cobalt acetate and 0.25 mmol of copper acetate, add them to the above mixed solution, and continue stirring to dissolve.
[0061] Add 0.7 mL of 25% aqueous ammonia dropwise.
[0062] The mixed solution was transferred to a 100 mL hydrothermal reactor and placed in an oven at 150 °C for 24 h.
[0063] The hydrothermal reaction product was centrifugally washed with deionized water and ethanol three times each, and placed in a vacuum drying oven at a temperature of 60 °C with a vacuum degree of -0.1 MPa.
[0064] The dried material was placed in a muffle furnace at 450 °C for 3 h to obtain copper cobaltate.
[0065] Step 2, the copper cobaltate o...
Embodiment 3
[0070] A nickel-cobaltate-loaded sulfur-lithium-sulfur battery composite cathode material, specifically prepared according to the following steps:
[0071] Step 1, nickel cobaltate (NiCo 2 o 4 ) preparation:
[0072] Measure 10 mL of deionized water and 40 mL of absolute ethanol and mix evenly.
[0073] Weigh 0.5 mmol of cobalt acetate and 0.25 mmol of nickel acetate, add them to the above mixed solution, and continue stirring to dissolve.
[0074] Add 1 mL of 25% aqueous ammonia dropwise.
[0075] The mixed solution was transferred to a 100 mL hydrothermal reactor and placed in an oven at 200 °C for 24 h.
[0076] The hydrothermal reaction product was centrifugally washed with deionized water and ethanol three times each, and placed in a vacuum drying oven at a temperature of 60 °C with a vacuum degree of -0.1 MPa.
[0077] The dried material was placed in a muffle furnace at 500 °C for 3 h to obtain nickel cobaltate.
[0078] Step 2, the nickel cobaltate obtained in st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com