Specific activity and heat stability improved glucose oxidase mutant and encoding gene and application of glucose oxidase mutant
A technology of glucose oxidase and thermal stability, applied in the field of genetic engineering, can solve the problems of poor thermal stability, limited industrial application, low specific activity, etc., and achieve the effects of reducing production cost and improving specific activity and thermal stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1, optimization and gene synthesis of Aspergillus niger (Aspergillus niger) glucose oxidase (GOD) gene
[0032] The glucose oxidase gene (Genebank: FJ979866.1) of Aspergillus niger GIM 3.452 was optimized for the codon preference of Pichia pastoris, and the optimized nucleotide sequence was synthesized by Suzhou Jinweizhi Biotechnology Co., Ltd. The 5' end and 3' end of the enzyme optimization gene (GOX) were introduced into EcoRI and XbaI restriction sites, and connected to the Puc57-amp vector, GOX-Puc57 was inoculated into LBA medium, and after culturing for 24 hours, the plasmid was extracted and used EcoRI and XbaI enzyme digestion, gel cutting to recover target gene fragments, product purification and recovery, were connected to expression vectors pPICzαA and pPGAPzαA, respectively, to obtain expression vectors pPICzαA-GOX and pGAPzαA-GOX.
Embodiment 2
[0033] Embodiment 2, site-directed saturation mutation
[0034] Using the above pPICzαA-GOX as a template and using the primers in the table for PCR amplification, the specific amplification reaction system is as follows:
[0035] Q5 High Fidelity Taq Enzyme MIX
23uL
Corresponding mutant primer 1 (50uM)
1uL
Corresponding mutant primer 1 (50uM)
1uL
pPICzαA-GOX (20ng)
2uL
Add water to
50uL
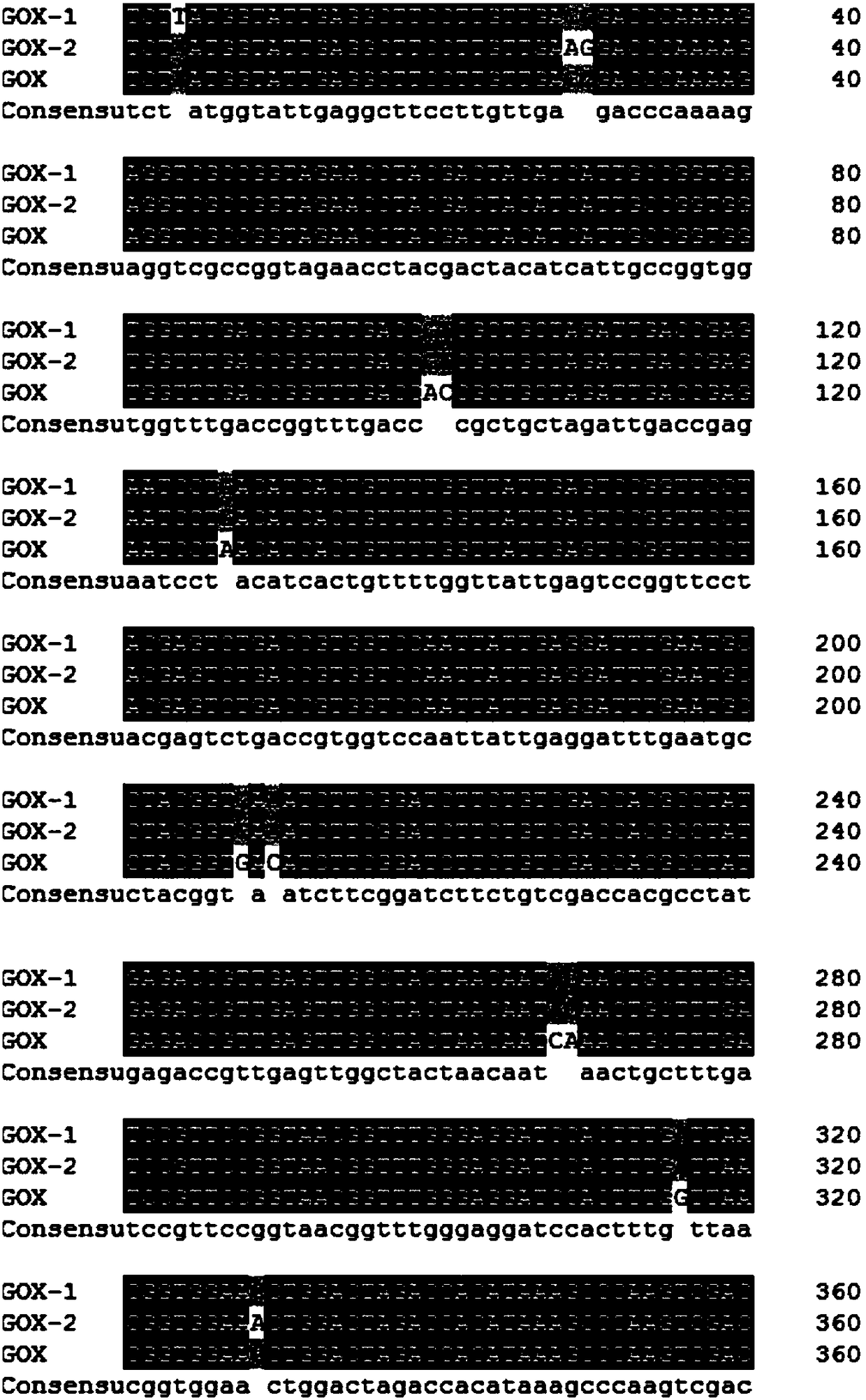
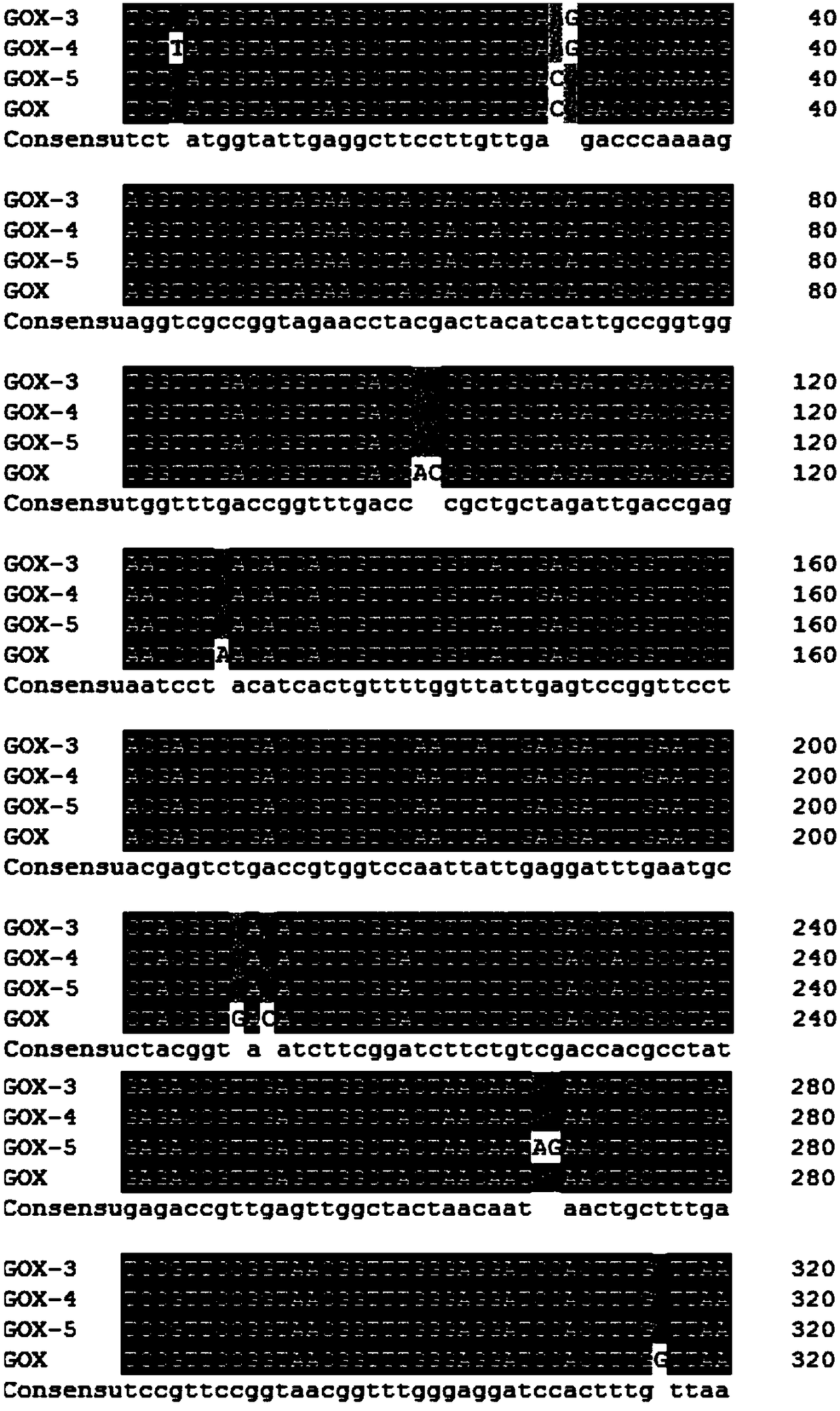
[0036] When the mutation site is V20Y, the corresponding mutant primer 1: 5'-cgccggtagaacctacgactacatcattgc-3'; the corresponding mutant primer 2: 5'-gcaatgatgtagtcgtaggttctaccggcg-3'. When the mutation site is T34V, the corresponding mutant primer 1: 5'-gaccggtttgaccgttgctgctagattga-3'; the corresponding mutant primer 2: 5'-tcaatctagcagcaacggtcaaaccggtc-3'. When the mutation site is D70Q, the corresponding mutant primer 1: 5'-ggatttgaatgcctacggtcagatcttcggatcttctgtcg-3'; the corresponding mutant primer 2: 5'-cgacagaagatccgaagatc...
Embodiment 3
[0039] Embodiment 3, high-throughput screening high specific activity mutant strain
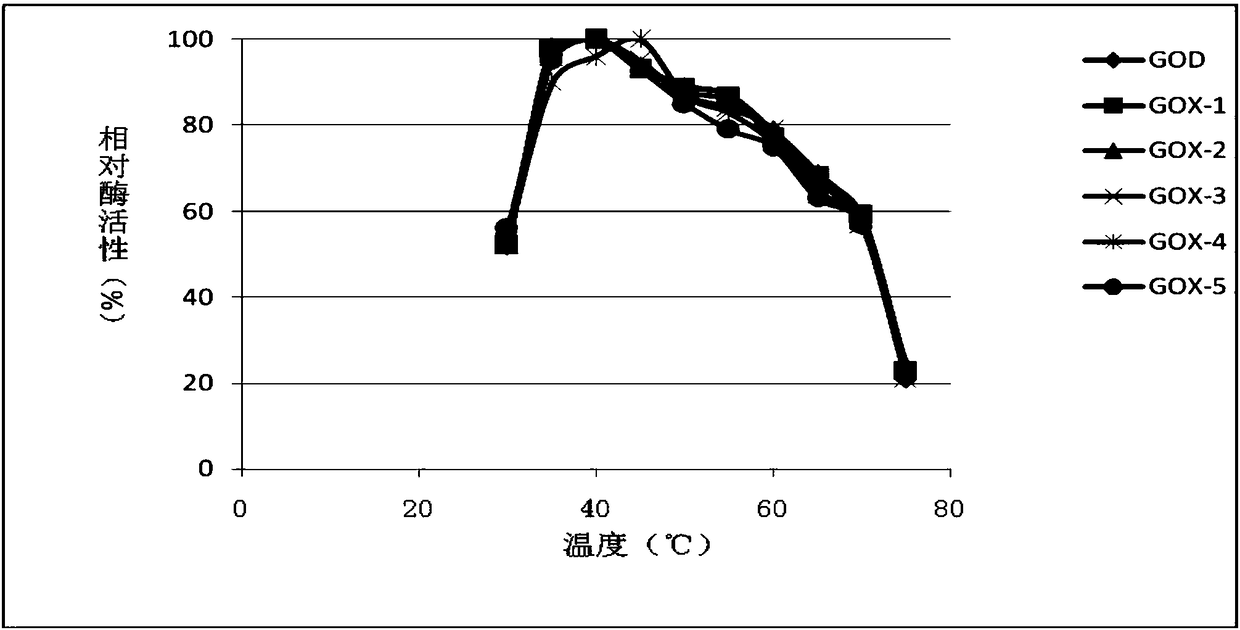
[0040] Pick the recombinant yeast transformants obtained in Example 2 to a 24-well plate one by one with a toothpick, add 1 mL of medium containing BMGY to each well, culture at 30° C., 220 rpm for about 24 hours, and centrifuge to remove the supernatant. Then add 1.6mL BMMY medium respectively for induction culture. After culturing for 24 hours, the supernatant was collected by centrifugation, and 200 μL of the above supernatant was taken out to a 96-well plate, and the enzyme activity of glucose oxidase was measured. After high-throughput screening, six effective mutation sites were obtained, namely V20Y, T34V, D70Q, Q90R, V106I, and Y509E. The relative specific activities of these six mutants are shown in Table 1.
[0041] Table 1 Relative specific activity of original glucose oxidase and mutant glucose oxidase
[0042] Numbering
[0043] It can be known from Table 1 that the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com